Guru Reddy1, Sanjay Mourya1, Steve Hasal1, Shalabh Gupta1
1Unicycive Therapeutics, Inc., Los Altos, CA
Background
- Acute kidney injury (AKI) is a clinical syndrome defined by the sudden loss of kidney function1, resulting in an inability to maintain electrolyte, acid-base and water balance2,3
- Currently, there are no effective treatments for AKI approved by the US Food and Drug Administration or the European Medicines Agency; management of the condition is primarily supportive2,4
- The most common cause of AKI was thought to be ischemia3
- However, more recently the focus has shifted from clinical causes to the underlying cellular processes inherent in these situations, in particular the dysfunction of mitochondria in AKI4
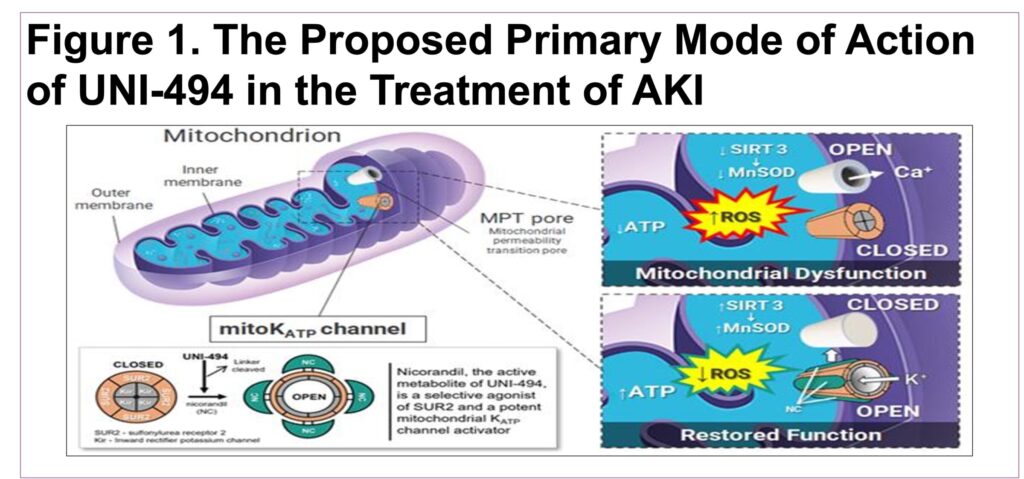
- UNI-494 is a selective mitochondrial ATP-sensitive potassium (KATP) channel activator that binds to the KATP channels, which reverses the mitochondrial dysfunction by closing the mPTP (Figure 1)
Methods
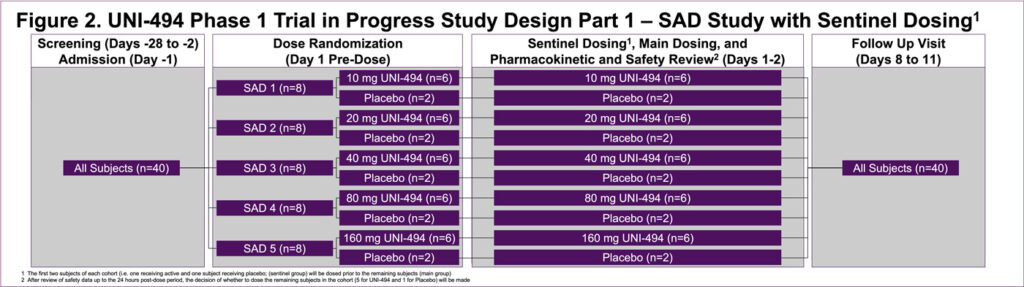
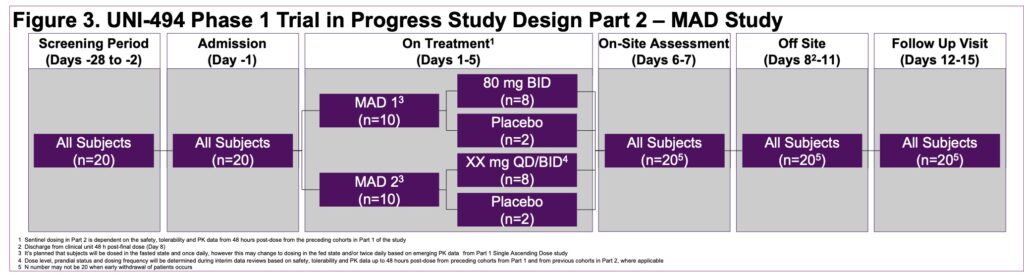
- This is a single-center, double-blind, placebo-controlled, randomized single ascending dose (SAD) (Part 1) (Figure 2) and multiple ascending dose (MAD) (Part 2) (Figure 3) study in healthy male subjects and female subjects of non-childbearing potential
- Part 1 will enroll up to approximately 40 subjects in 5 cohorts of 8 subjects each (randomized to a ratio of 6 active and 2 placebo per cohort) (Figure 2)
- There will be an interim decision meeting after each cohort/period, to review the safety, tolerability, and pharmacokinetics (PK) data up to 48 hours post-dose in order to decide the dose level for the subsequent cohort
- Part 2 will enroll approximately 20 subjects in 2 cohorts of 10 subjects each, randomized to a ratio of 8 active treatment to 2 placebo who will be dosed for 5 days (Figure 3)
- The dose level for the Part 2 Cohort 1 (80 mg BID) was selected based on the safety, tolerability, and PK data from Part 1

CONCLUSIONS
- The safety, tolerability, and PK of UNI-494 in healthy subjects will be evaluated in this study
- We hope to show results by 2H 2024
References:
1. Lv JC, Zhang LX. Adv Exp Med Biol. 2019.
2. Am J Kidney Dis. 2003.
3. King AJ, et al. Sci Transl Med. 2018.
4. King AJ, et al. Am J Physiol Renal Physiol. 2021.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.