Guru Reddy1, PhD; Sanjay S. Mourya1; Steve Hasal1, PhD; Shalabh Gupta1, MD
1Unicycive Therapeutics, Inc., Los Altos, CA
Background
- Currently, there are no effective treatments approved for acute kidney injury (AKI)1,2
- Inflammation and reactive oxygen species driven mitochondrial permeability transition pore (mPTP) opening causes mitochondrial dysfunction/swelling and cell death3-7
- This is implicated in acute diseases originating from ischemia reperfusion injury or delayed graft function (DGF)3-7
- Furthermore, unresolved inflammation exacerbates sustained mPTP opening, evident in chronic kidney diseases8,9
- UNI-494 is a selective mitochondrial ATP-sensitive potassium channel (KATP) activator which reverses the mitochondrial dysfunction by closing mPTP

Objective
We present results from a phase 1 study evaluating safety, tolerability, and pharmacokinetics (PK) of UNI-494 capsules administered to healthy subjects.
Methods
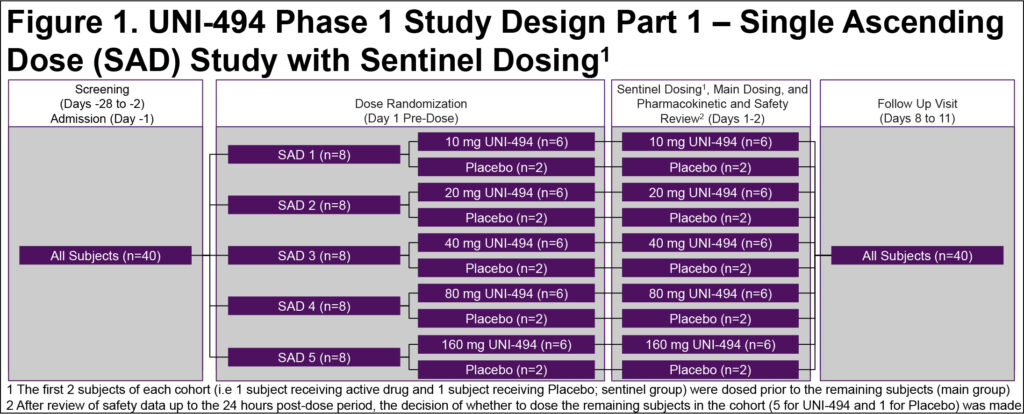
- This was a single-center, double-blind, placebo-controlled, randomized single ascending dose (SAD) study in healthy males and females of non-childbearing potential
- Study enrolled up to 40 subjects in 5 cohorts of 8 subjects each (6 active/2 placebo per cohort) (Figure 1)
- There was an interim decision meeting after each dose cohort to review the safety, tolerability, and PK data up to 48 h post-dose to decide the dose level for the subsequent cohort
- Safety assessments and systemic exposure to UNI-494 and its metabolites (nicorandil and CHEA) were conducted
Results
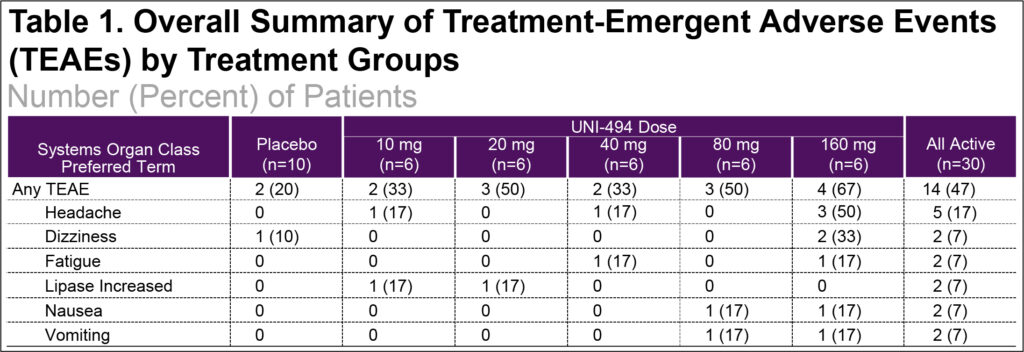
- Following single oral administration of 10, 20, 40, 80, and 160 mg UNI-494 capsules, most treatment-emergent adverse events (TEAEs) were mild severity (93%), there were no severe TEAEs, and no subjects were withdrawn for adverse events (Table 1)
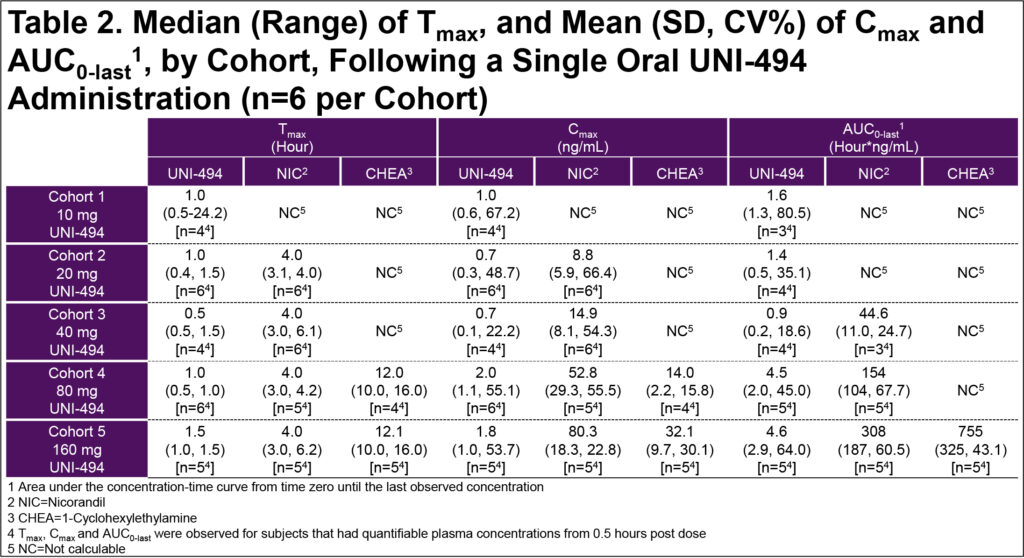
- In the SAD cohorts, overall exposures to UNI-494 were low due to rapid conversion to nicorandil; mean nicorandil Cmax was 14.9, 52.8, and 80.3 ng/mL and mean AUC(0-last) was 44.6, 154, and 308 hour*ng/mL for the 40, 80, and 160 mg UNI-494 dose groups, respectively (Table 2)

Conclusions
- Single dose of 10-160 mg of UNI-494 capsules were safe and well-tolerated in healthy volunteers
- UNI-494 was rapidly converted to nicorandil
- Exposure to nicorandil increased in a dose-proportional manner
- Therapeutic levels (AUC >200 hour*ng/mL) of nicorandil achieved at 60 mg UNI-494
Discussion
- The rapid conversion of UNI-494 to nicorandil and 1-cyclohexylethylamine indicates a potential for a fast-acting therapy for the prevention of DGF and other AKI clinical conditions
- Future studies should evaluate this promising treatment in the target population of patients with AKI
References
1. Mercado MG, et al., Am Fam Physician. 2019.
2. Szeto HH., J Am Soc Nephrol. 2017.
3. de Mello AH, et al., Life Sciences. 2018.
4. Sharov VG, et al., J Mol Cell Cardiol. 2007.
5. Mishra J, et al., Cells. 2019.
6 Kinnally KW, et al., Biochim Biophys Acta. 2011.
7. Endlicher R, et al., Cells. 2023.
8. Kent A., Oxidative Medicine and Cellular Longevity. 2021.
9. Irazabal MV., Cells. 2020.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.