Vandana Mathur, MD, FASN 1 , ∗ , Michael Walker, PhD 2 ,Steve Hasal, PhD 3 ,Guru Reddy, PhD 3 , Shalabh Gupta, MD 3
1 Mathur Consulting, LLC, Woodside, CA
2 Independent Consultant, Biostatistics, San Diego, CA
3 Unicycive Therapeutics, Inc., Los Altos, CA
Abstract
Background
Phosphate binders (PB) are integral to hyperphosphatemia management in patients with end-stage kidney disease. PB efficacy is adversely affected by nonadherence and limited phosphate-binding capacity relative to dietary intake. Oxylanthanum carbonate is an investigational novel nanotechnology product that combines lanthanum, which has the highest binding capacity of available PBs, with a smaller pill size that is swallowed with water rather than chewed. This study’s objective was to demonstrate the pharmacodynamic equivalence of orally administered oxylanthanum carbonate to lanthanum carbonate (LC) in healthy subjects.
Methods
In this phase one, single-center, randomized, open-label study, healthy subjects were treated with oxylanthanum carbonate swallowable tablets 1000 mg three times/day and LC chewable tablets 1000 mg three times/day in a two-way crossover design. The primary pharmacodynamic variable was the least squares mean (LSM) change in urinary phosphate excretion from baseline to the evaluation period (Days 1–4 of treatment).
Findings
A total of 80 subjects were randomized and 75 received all doses. The LSM change in urinary phosphate excretion from Baseline to the Evaluation (Treatment) Period was similar for both oxylanthanum carbonate (–320.4 mg/day [90% CI: –349.7, –291.0]) and LC (–324.0 mg/day [90% CI: –353.3, –294.7]); the between-group LSM difference was 3.6 [90% CI: –37.8, 45.1] mg/day. Both drugs were well tolerated with an equal incidence of adverse events.
Implications
Thus, oxylanthanum carbonate was bioequivalent to LC in healthy subjects and well tolerated. Oral oxylanthanum carbonate may provide an option for patients with chronic kidney disease and hyperphosphatemia for whom chewing tablets is disliked, inconvenient, or difficult. Clinical Trial Registration Number: NCT06218290.
Key words
Bioequivalent, Chronic kidney disease, Hyperphosphatemia, Lanthanum, Phosphate

Introduction
End-stage kidney disease (ESKD) affects more than seven million people worldwide. 1 Patients with chronic kidney disease (CKD) retain phosphorus due to reduced glomerular filtration. 2 The incidence of hyperphosphatemia increases with progressive reduction in the estimated glomerular filtration rate (eGFR) below 40 mL/min/1.73 m 3 ; once patients have reached ESKD, approximately 70% have hyperphosphatemia. 4 According to the Dialysis Outcomes and Practice Patterns Study Practice Monitor, which reports data from a national sample of United States hemodialysis patients, the most recent serum phosphate concentration for the reporting period (February 2021) was > 5.5 mg/dL in 43.1% of patients and > 4.5 mg/dL in 71.2% of patients. 5
In patients with CKD G3a through G5D, representing a spectrum of patients with reduced eGFR not yet on dialysis to those with kidney failure on dialysis, 6 current Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend treatments for hyperphosphatemia such as dietary intervention, phosphate binders (PB), and dialysis to lower elevated serum phosphate concentrations toward the normal range while avoiding hypercalcemia (calcium levels above 10.2 mg/dL [ > 2.55 mmol/L]). 7 These recommendations were based, in part, on a consistent association between increasing serum phosphate and all-cause mortality in most studies examining this relationship. In one such study of over 6000 patients with nondialysis-dependent CKD, each one mg/dL increase in serum phosphate was associated with an estimated 25% increased risk of all-cause mortality. 8
Despite the guidance from KDIGO, > 40% of United States patients receiving hemodialysis had phosphate concentrations above > 5.5 mg/dL in their most recent measurement, 5 which, although reflecting a single timepoint, is likely to not represent “approaching the normal range. ”Potential barriers to effective phosphate lowering include low adherence to prescribed treatments 9 and insufficient phosphate binding capacity to match dietary intake. 10 With respect to PB specifically, high pill burden due to the need to take multiple large pills and adverse gastrointestinal effects may decrease adherence, consequently reducing effectiveness. 9 , 11
Oxylanthanum carbonate is an investigational new drug being developed under FDA’s 505(b)(2) regulatory pathway. If approved, oxylanthanum carbonate will share substantially the same product label and prescribing information as the reference-listed drug Fosrenol®(lanthanum carbonate [LC]) with the exception that oxylanthanum carbonate tablets are smaller in size and swallowed whole with water rather than chewed. Oxylanthanum carbonate is a novel nanotechnology product that contains lanthanum, which has the highest binding capacity of available PB. 12 Further, a significantly lower volume of oxylanthanum carbonate is needed to bind an equivalent amount of phosphate compared to LC (2.3 cm3 vs 8.0 cm3 ). 13 Thus, smaller oxylanthanum carbonate tablets can likely bind an equivalent amount of phosphate than the larger LC tablets, potentially improving effectiveness, adherence, and patient quality of life. The objective of this study was to demonstrate the pharmacodynamic equivalence of 1000 mg thrice-daily (TID) oral (swallowed) oxylanthanum carbonate to 1000 mg TID oral (chewed) LC in healthy subjects.
Participants and Methods
Overall Study Design
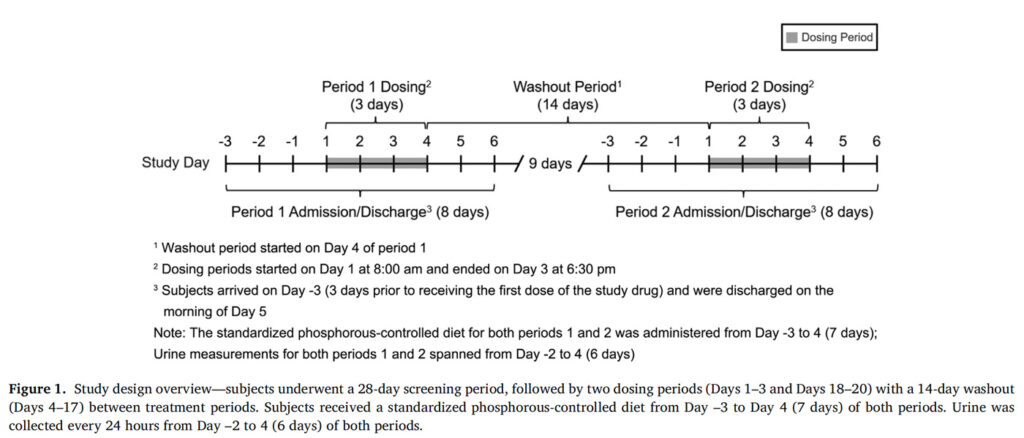
A phase 1, single-center, randomized, open-label, two-way crossover study in normal healthy subjects was conducted from August 2022 to October 2022 to establish pharmacodynamic equivalence between oxylanthanum carbonate swallowable tablets 1000 mg TID and LC chewable tablets 1000 mg TID. Subjects were randomized to the order of treatment with the two study drugs (oxylanthanum carbonate [Period 1] then LC [Period 2] or LC [Period 1] then oxylanthanum carbonate [Period 2]) using a computer-generated randomization scheme. The study consisted of a Screening Period (28 days before Day –4 of Period 1), two dosing periods separated by a 14-day washout period, and a follow-up period of seven days after the last study drug dose ( Figure 1 ). Study subjects were housed in an inpatient clinical research unit from Day –3 to Day 5 of each treatment period. Subjects received a standardized phosphorous-controlled diet from Day –3 to Day 4 of each study period. This diet was designed to contain a total phosphorus content of approximately 1400 mg/day, with a total calorie content of approximately 2500 kcal/day, divided approximately equally between the three daily meals. The planned meals were also devised to provide similar daily calcium intake (770–890 mg). The dietary phosphorus content selected for this study was chosen to be consistent with the mean consumption of this mineral in the National Health and Nutrition Examination Survey population (1399 [95% CI: 1376,1423] mg/day). 14 Because intestinal absorption of phosphate differs by phosphate source, 15 the study diet aimed to maintain constancy in the percentage of phosphorous from animal, plant, and inorganic sources on each day. Urine was collected for 24 hours on five consecutive days during each study period, beginning on Day –2. The central laboratory (Charles River Laboratory Edinburgh LTD, Edinburgh, United Kingdom) performing analysis of urine phosphate was blinded to treatment assignment. Urine phosphate results were not accessible to the study site at any time during the study.
Figure 1. Study design overview
Participants
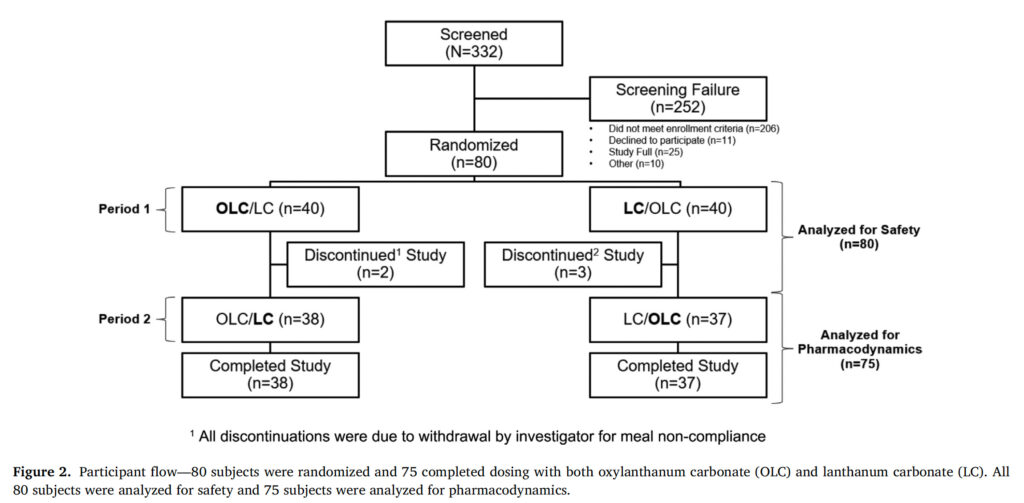
The study aimed to enroll a sufficient number of subjects to achieve 64 evaluable healthy volunteer subjects between the ages of 18 and 55 years (inclusive; see Table SI for a full list of eligibility criteria). 332 subjects were screened and 80 were randomized to receive oxylanthanum carbonate or LC ( n = 40 for each group) ( Figure 2 ).
Figure 2. Participant Flow
Ethics Approval
The study protocol and the written informed consent form were reviewed and approved by an Institutional Review Board (Advarra, Columbia, MD) as required by the US Code of Federal Regulations (21 CFR 56). The study was conducted in accordance with Good Clinical Practice, the US FDA Code of Federal Regulations 21 CFR 312, 21 CFR Parts 50 and 46, and the World Medical Association Declaration of Helsinki. A written informed consent was signed by each subject prior to any study-related assessments or procedures.
Interventions
Each oxylanthanum carbonate tablet contained 1000 mg of lanthanum as oxylanthanum carbonate and inactive ingredients. Commercially available Fosrenol®(LC) Chewable Tablets 1000 mg were used as the active comparator. The study drugs (1000 mg oxylanthanum carbonate or 1000 mg LC) were administered by the study staffone to 10 minutes after breakfast, lunch, and dinner on Days 1 to 3 of each study period. The study staffobserved subjects to ensure that LC had been fully chewed and that all tablets of oxylanthanum carbonate had been swallowed.
Outcome Measures
The primary pharmacodynamic outcome was the least squares mean (LSM) change in 24-hour urinary phosphate excretion from the Baseline Period to the Evaluation Period. The Baseline Period was defined as the approximately 48-hour urine collection period starting on Day –2 and ending on Day 1. The Evaluation Period was defined as the approximately 72-hour urine collection period starting on Day 1 and ending on Day 4.
Safety assessments included the incidence, frequency, and severity of adverse events. Adverse events were classified according to the terminology of Medical Dictionary for Regulatory Activities (MedDRA Version 25.0) by preferred term and system organ class (SOC).
Analytical Methods
The sample size was estimated based on pooled data of reductions in urinary phosphate excretion observed in previous studies of LC. At a significance level of 0.05, in a two-way crossover study design assuming an expected treatment difference of 1.1 mmol/day for change in urinary phosphate excretion and pooled standard deviation for the mean change from baseline of 6.25 mmol/day, 32 subjects per treatment sequence were estimated to provide a statistical power of 90% for two one-sided equivalence tests.
The safety population, which was used to summarize safety data, was prespecified as all randomized subjects who received at least one dose of the study drug. The pharmacodynamic population, which was used to summarize the primary outcome measure, was prespecified as all subjects who completed all urine collection periods, had no important protocol deviations with respect to consumption of the standardized diet meals, and had taken all nine doses of study drug in both periods.
The primary pharmacodynamic variable was assessed using a mixed-effect linear model with fixed effects for sequence, period, and study drugs; random effect for individual within-sequence, and baseline as a covariate. Based on the mixed-effect linear model, a standard 90% CI was constructed for the difference in LSM of change in urinary phosphate excretion from the Baseline Period to the Evaluation Period. In addition, a reference interval (acceptance range) for the treatment (effect) was defined as ± 20% of the LSM of the primary pharmacodynamic variable for LC. Additional details on the statistical model are included in the supplement (see also Figures S1 and S2).
For safety analyses, descriptive statistics included the percentage of observations in various categories. SAS®Version 9.4 (or higher) was used for analyses and sample size estimations.
Results
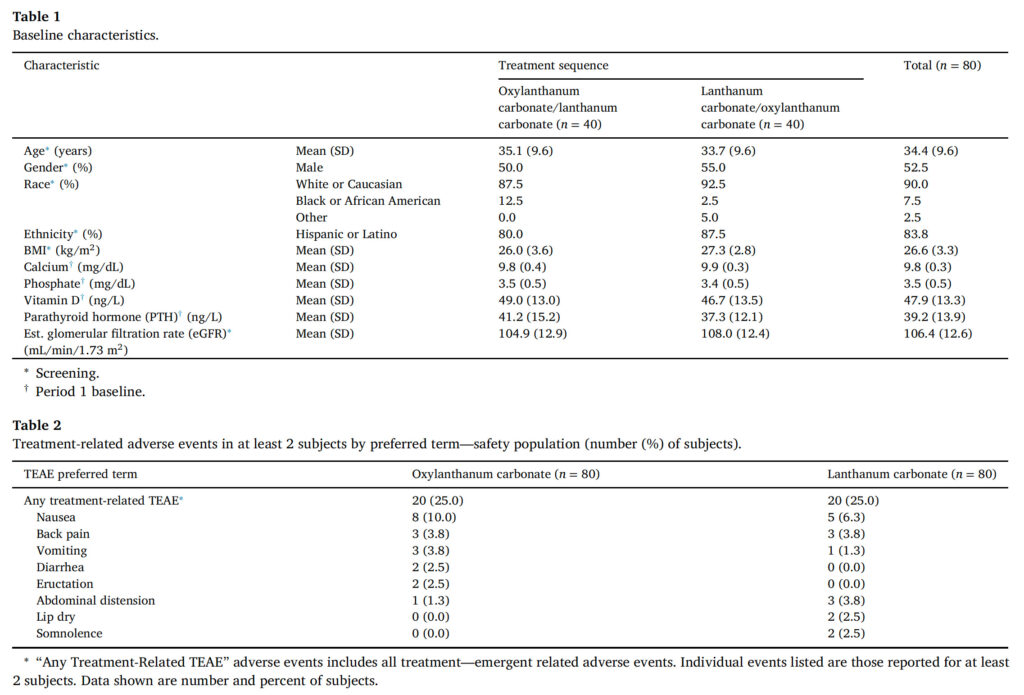
A total of 80 subjects were randomized and 75 received all doses of both study drugs (oxylanthanum carbonate and LC). All 80 subjects were included in the safety population and 75 were included in the pharmacodynamic population ( Figure 2 ). Baseline characteristics were balanced between the oxylanthanum carbonate/LC and LC/oxylanthanum carbonate sequences ( Table 1 ). The LSM (SE) phosphate excretion at baseline was 861.6 (30.9) mg/day in the oxylanthanum carbonate group and 876.1 (30.9) mg/day in the LC group (Table SII).
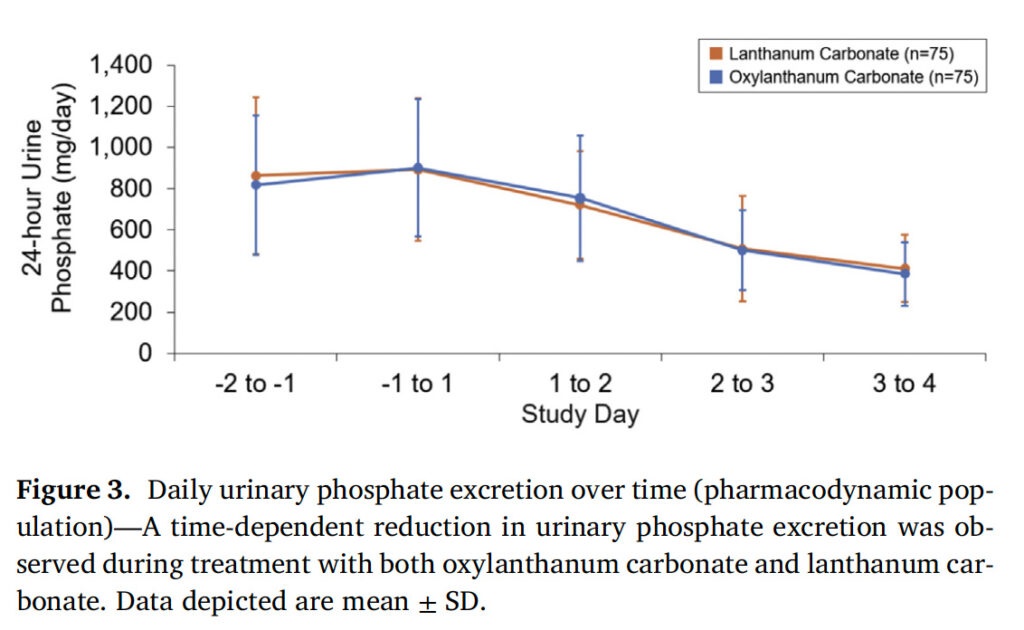
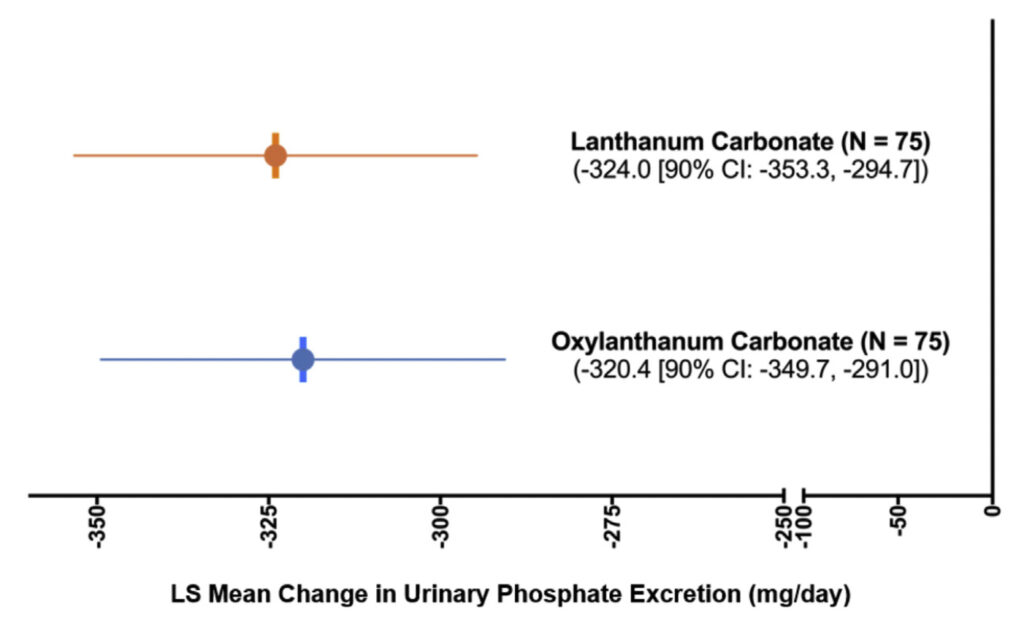
There was a time-dependent reduction in 24-hour urine phosphate excretion during the treatment period (Day 1 to Day 3) in both treatment groups ( Figure 3 ). The LSM changes from baseline to the evaluation period in urinary phosphate excretion during treatment with oxylanthanum carbonate and LC were –320.4 mg/day [90% CI: –349.7, –291.0] and –324.0 mg/day [90% CI: –353.3, –294.7], respectively ( Figure 4 , Table SII). The treatment effect (oxylanthanum carbonate –LC) was 3.6 mg/day [90% CI: –37.8, 45.1], which was within the bounds of the acceptance range ( ± 20% of the reference [LC] LSM change from baseline [–64.8, 64.8]) (Table SII).
Figure 3. Daily urinary phosphate excretion over time
There were no serious or severe adverse events, or treatmentemergent adverse events (TEAEs) resulting in treatment discontinuation or interruption. The percentages of subjects with any adverse event during the oxylanthanum carbonate and LC periods were equal (35.0%). By body system, adverse events coded to the Gastrointestinal Disorders SOC were most common during both the oxylanthanum carbon- ate (11 [13.8%] subjects) and LC (15 [18.8%] subjects) periods. None of these TEAEs required treatment. All gastrointestinal system adverse events were mild in severity other than two events reported as moder- ate: toothache (oxylanthanum carbonate, one subject) and constipation (LC, one subject).
Figure 4. Change in urinary phosphate excretion from base- line to the evaluation period
The incidence of treatment-related TEAEs was the same for oxylan- thanum carbonate and LC (25.0%) ( Table 2 ) and the majority of these were mild in severity. The highest incidence of treatment-related TEAEs was in the Gastrointestinal Disorders SOC for both oxylanthanum car- bonate (13.8%) and LC (18.8%). The most common of these was nausea, which was reported in eight (10%) subjects treated with oxylanthanum carbonate and five (6.3%) subjects treated with LC; all reports of nau- sea were assessed as mild in severity. Subjects were not treated for any of these events and there was no action taken with the study drug by the investigator. Back pain, vomiting, and dry skin were all reported in three (3.8%) subjects during oxylanthanum carbonate treatment. Back pain, abdominal distension, and headache were each reported in three (3.8%) subjects during LC treatment. All other treatment-related TEAEs were reported in ≤ two subjects during oxylanthanum carbonate and LC treatment.
Table 1 and 2

DISCUSSION
In this randomized, two-way crossover design study, oxylanthanum carbonate and LC were found to be bioequivalent in lowering urinary phosphate excretion in healthy volunteers. While determination of drug bioequivalence is generally done by utilizing pharmacokinetic measurements, systemic oral bioavailability of lanthanum is minimal. 16 , 17 Thus, a bioequivalence approach for nonabsorbed drugs based on pharmacodynamic endpoints, which is supported by regulatory guidance, was used in this study. 18 United States Food and Drug Administration guidelines specific to LC recommend demonstration of bioequivalence in healthy subjects with change in urinary phosphate as an endpoint. 18 Most patients on dialysis and a minority of patients with CKD not on dialysis receive treatment for hyperphosphatemia. 19 , 20 Despite these treatments, many do not approach normal phosphate levels. 5 The average overall pill burden for patients on dialysis is 19 pills per day and approximately half of this burden arises from PB medications. 11 Nonadherence related to pill burden and other undesirable-to-patients attributes of PB likely reduces their effectiveness. 9 Lanthanum has the highest phosphate binding capacity of available PB 12 and thus has the potential to be less burdensome in terms of number of required pills. However, chewable LC tablets may result in variable efficacy related to completeness of chewing. 21 Oxylanthanum carbonate tablets are swallowed whole, thus removing the need for patients to completely chew the tablets to achieve the full effect of the treatment. The strengths of this study included the randomized crossover design, tight control of dietary variability through the use of a study diet standardized for phosphorus, calcium, and calories, as well as collection of timed urine specimens, administration of study drug under direct supervision in an inpatient setting, and reasonable demographic diversity of the study population in terms of sex, race, and ethnicity. Limitations of this study included its open-label design. Several aspects mitigated the risk of bias from the open-label design: (1) the primary outcome was based on an objective measurement (urinary phosphate); (2) the analysis of urine phosphate was conducted at a laboratory blinded to treatment assignment; and (3) the study site did not receive any urine phosphate results. Additionally, because the study was conducted in a healthy volunteer population (per regulatory guidance 18 ) rather than in a CKD population, the study does not directly demonstrate safety or efficacy in patients with CKD. Although the majority of patients with ESKD in the United States are managed with PB, the optimal strategies for garnering the greatest effectiveness from these drugs while minimizing pill burden and adverse effects for patients are yet to be defined. In this study, we demonstrated the bioequivalence of a novel nanotechnology product, oxylanthanum carbonate, with LC. The higher intrinsic phosphate binding capacity of lanthanum without the potential variability introduced by the need to completely chew tablets may provide an additional option for patients with hyperphosphatemia for whom chewing tablets is disliked, inconvenient, or difficult.
CONCLUSIONS
Oxylanthanum carbonate was found to be bioequivalent to LC and well tolerated in healthy subjects. A PB with the high intrinsic phosphate binding capacity of lanthanum and without the potential variability in efficacy introduced by the need to completely chew tablets may provide an additional option for patients with hyperphosphatemia for whom chewing tablets is disliked, inconvenient, or difficult.
Declaration of competing interest
Steve Hasal, Guru Reddy, and Shalabh Gupta are employees of Uni- cycive Therapeutics, Inc. Vandana Mathur and Michael Walker are consultants for Unicycive Therapeutics, Inc. Editorial support was funded by Unicycive Therapeutics, Inc.
CRediT authorship contribution statement
Vandana Mathur: Conceptualization, Methodology, Writing –re- view & editing.Michael Walker: Methodology, Data curation, Formal analysis, Writing –review & editing.Steve Hasal: Methodology, Writ- ing –review & editing.Guru Reddy: Conceptualization, Methodology, Writing –review & editing.Shalabh Gupta: Conceptualization, Method- ology, Writing –review & editing.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author [md@mathurconsulting.com] will on request detail the restrictions and any conditions under which access to some data may be provided.
Acknowledgments
Editorial support was provided by Xelay Acumen Group, Inc. All the authors have authorized the submission of their manuscript via Xelay Acumen Group, Inc. and have approved all statements and declarations, including conflicting interests and funding.
Funding
This work was supported by Unicycive Therapeutics, Inc .
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clinthera.2024.11.009 .
References:
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol . 2019;1165:3–15
- Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated be-fore parathyroid hormone and phosphate in chronic kidney disease. Kidney Int . 2011;79:1370–1378
- Levin A, Bakris GL, Molitch ME, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int . 2007;71(1):31–338 .
- National Kidney Foundation (NKF). K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis . 2003;42:S1–S201
- Serum phosphorus (most recent), categories. DOPPS practice monitor. 2023. https://www.dopps.org/DPM-HD/Files/phosphmgdl_c_overallTAB.htm . Down-loaded October 9. 2023
- How to Classify CKD. National kidney foundation. https://www.kidney.org/ professionals/explore-your-knowledge/how-to-classify-ckd . Downloaded May 29. 2024
- KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, preven-tion, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011) . 2017;7:1–59 .
- Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol . 2005;16:520–528
- Van Camp YP, Vrijens B, Abraham I, Van Rompaey B, Elseviers MM. Adherence to phosphate binders in hemodialysis patients: prevalence and determinants. J Nephrol . 2014;27:673–679 .
- Huml AM, Sullivan CM, Leon JB, Sehgal AR. The adequacy of phosphorus binder
prescriptions among American hemodialysis patients. Ren Fail . 2012;34:1258–1263 . - Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden,
adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients.
Clin J Am Soc Nephrol . 2009;4:1089–1096 . - Daugirdas JT, Finn WF, Emmett M, Chertow GM. The phosphate binder equivalent
dose. Semin Dial . 2011;24:41–49 . - Sprague SM, Reddy G, Jermasek D, Gupta P. High phosphate-binding capacity of oxylanthanum
carbonate with a low medication volume: comparison with commercially
available phosphate binders. Am J Nephrol . 2023;54:219–223 . - Watanabe MT, Barretti P, Caramori JCT. Attention to food phosphate and nutrition
labeling. J Ren Nutr . 2018;28:e29–e31 . - Bioavailability studies submitted in NDAs or INDs – general considerations. 2022.
https://www.fda.gov/media/121311/download . Downloaded 23 May. 2024 - Damment SJ, Pennick M. Clinical pharmacokinetics of the phosphate binder lanthanum
carbonate. Clin Pharmacokinet . 2008;47:553–563 . - Pennick M, Dennis K, Damment SJ. Absolute bioavailability and disposition of lanthanum
in healthy human subjects administered lanthanum carbonate. J Clin Pharmacol
. 2006;46:738–746 . - Draft guidance on lanthanum carbonate. 2017.
https://www.accessdata.fda.gov/
drugsatfda_docs/psg/Lanthanum%20Carbonate_draft_Oral%20tabs%20chewable_
RLD%2021468_RC05-17.pdf . Downloaded 23 May. 2024 - Berner T, Ferro C, Dieguez G, et al. Real-world phosphate binder use among dialysis-
dependent patients with CKD. Nephron . 2023;147:583–590 . - Liabeuf S, McCullough K, Young EW, et al. International variation in the management
of mineral bone disorder in patients with chronic kidney disease: results from
CKDopps. Bone . 2019;129:115058 . - Yang Y, Bykadi S, Carlin AS, Shah RB, Yu LX, Khan MA. Comparative evaluation
of the in vitro efficacy of lanthanum carbonate chewable tablets. J Pharm Sci .
2013;102:1370–1381 .