Atul Khare1, PhD; Pramod Gupta1, PhD; Guru Reddy1, PhD; Shalabh Gupta1, MD
1Unicycive Therapeutics, Inc.
Background
- Mitochondrial dysfunction in renal cells play a critical role in the pathophysiology of acute kidney injury (AKI) and chronic kidney disease (CKD)1
- Inflammation and reactive oxygen species (ROS) driven mitochondrial permeability transition pore (mPTP) opening causes mitochondrial dysfunction/swelling and eventual cell death over time
- This is implicated in a wide range of acute diseases including acute kidney disease (AKI) originating from ischemia reperfusion injury (IRI) or delayed graft function (DGF)
- Furthermore, unresolved inflammation exacerbates sustained mPTP opening, evident in chronic kidney diseases (CKD)
- UNI-494 is a selective mitochondrial ATP-sensitive potassium channel activator that binds to the ATP-sensitive potassium (KATP) channels which reverses the mitochondrial dysfunction by closing mPTP pore

OBJECTIVE
We present pharmacokinetic data in dogs for UNI-494
Methods
- Groups of 3 beagle dogs were administered a single oral dose of 3, 10, or 30 mg/kg UNI-494 at a volume of 5 mL/kg
- Clinical observations were recorded at approximately 1, 1.5, 2, 3, and 24h post-dose
- Whole blood samples were collected pre-dose and 0.083, 0.25, 0.50, 1, 1.5, 2, 4, 8, and 24h post-dose to analyze systemic exposure to UNI-494 and nicorandil
- Dose and concentration parameters (Cmax and AUC) were used to generate linearity plots and calculate the coefficients of determination (R2) and slopes for UNI-494 and nicorandil
Results
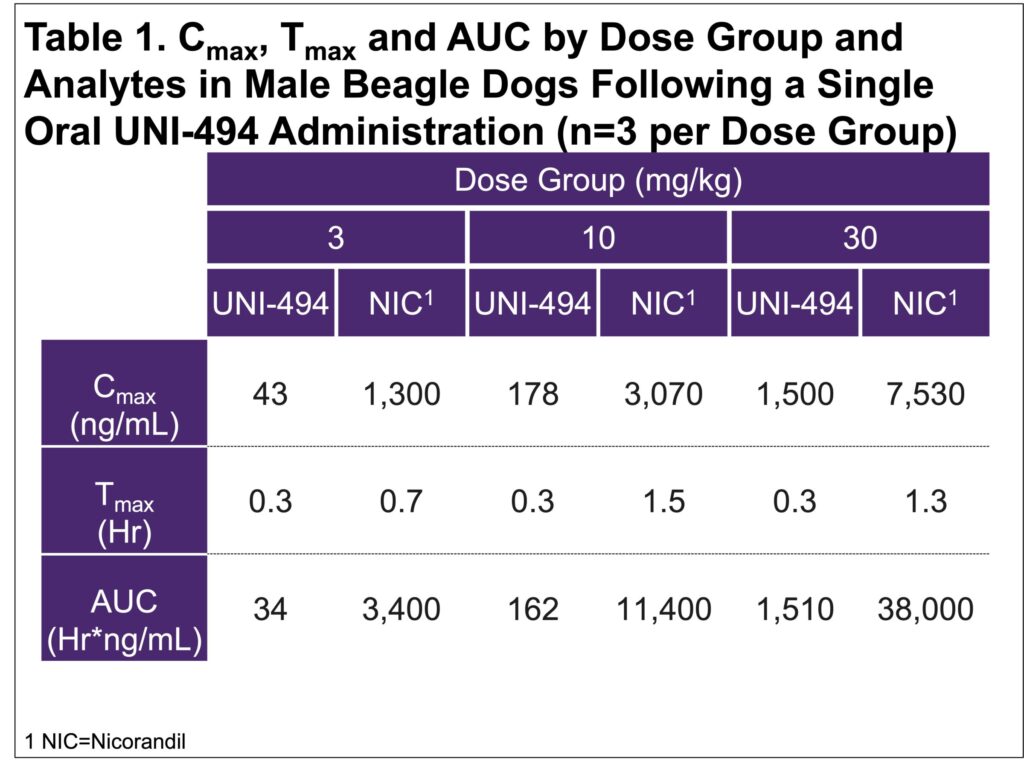
- The mean Tmax of nicorandil was 0.7h (3 mg/kg dose), 1.5h (10 mg/kg dose), and 1.3h (30 mg/kg dose), respectively (Table 1)
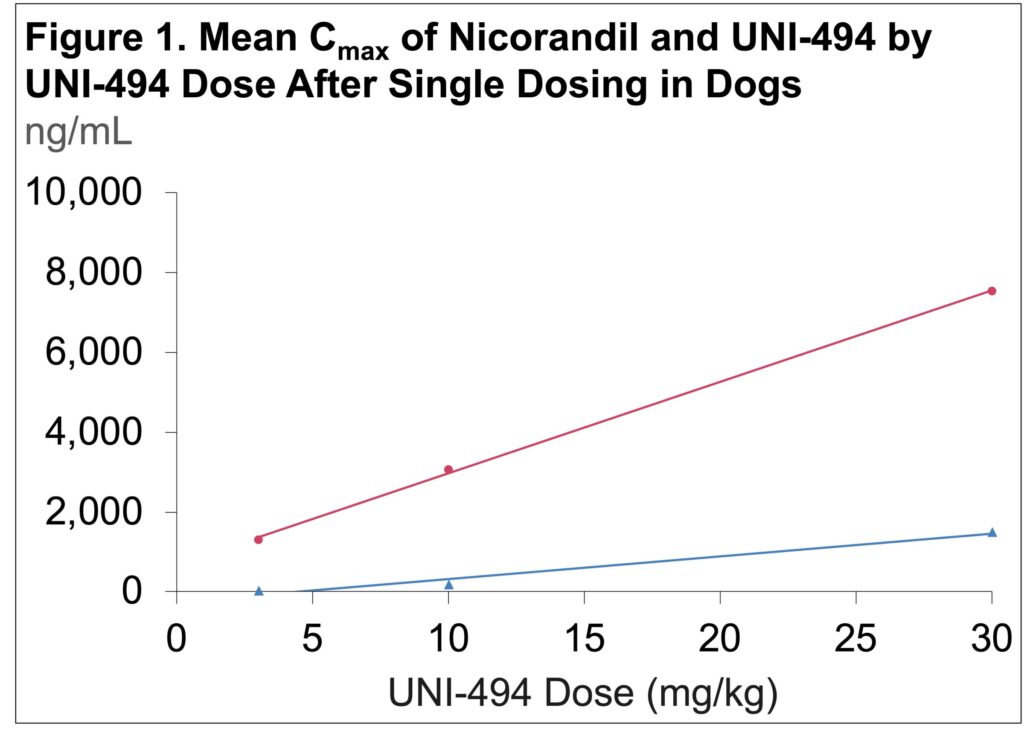
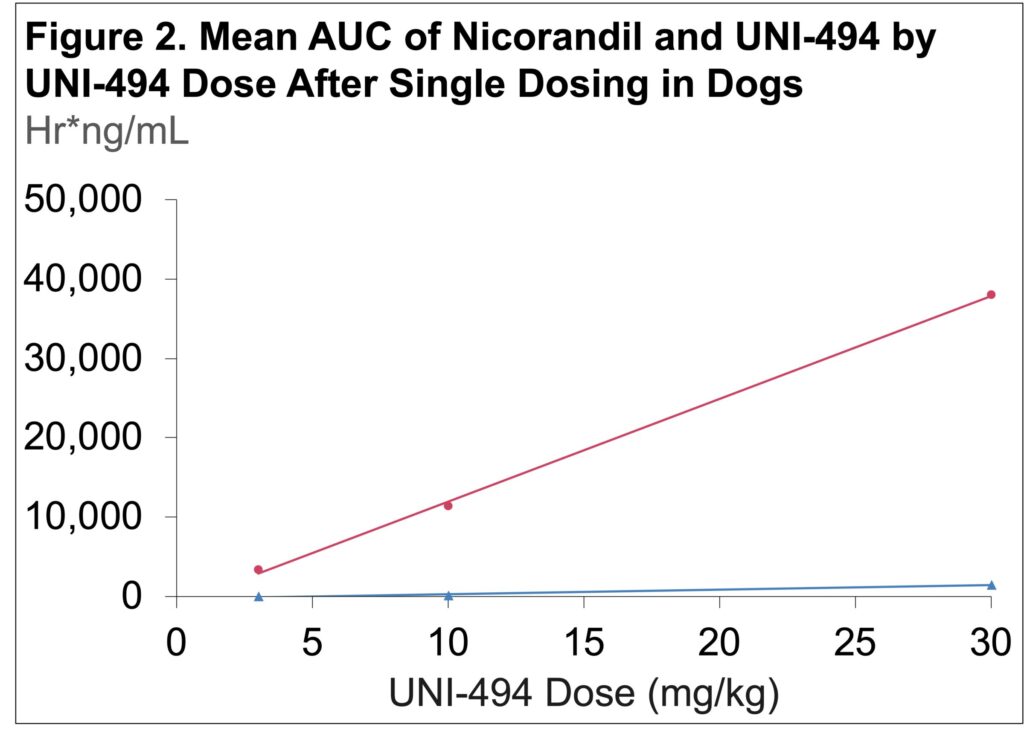
- The mean Cmax and AUC of nicorandil increased linearly with UNI-494 dose amounts (Figure 1 & 2)

CONCLUSIONS
- Nicorandil was rapidly formed from the prodrug UNI-494
- Mean Cmax for nicorandil was >5-fold greater than that of UNI-494, demonstrating the efficient conversion of the prodrug to the active drug
- The conversion was consistent across dose groups
DISCUSSION
- These results indicate that UNI-494 is a rationally designed drug
- Future studies should evaluate this promising treatment in the target population of patients with acute kidney injury
References:
1. Zhang X, et al. Int J Mol Sci. 2021..
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.