P.E.PERGOLA1, S. MOURYA2, S. HASAL2, G. REDDY2, S. GUPTA2
1 Renal Associates PA; 2 Unicycive Therapeutics, Inc.
Background
- Despite current therapies, more than 43% of patients undergoing dialysis in the USA have hyperphosphatemia, defined as serum phosphate values >5.5 mg/dL and is commonly associated with an increased risk of death1
- Most currently available phosphate binders are known to cause gastrointestinal (GI) adverse events (AEs) that can negatively impact the patient experience2
- Common GI AEs with phosphate binders include abdominal pain, nausea, and bloating
- Phosphate binders are usually formulated as large tablets, and some must be chewed before ingestion2
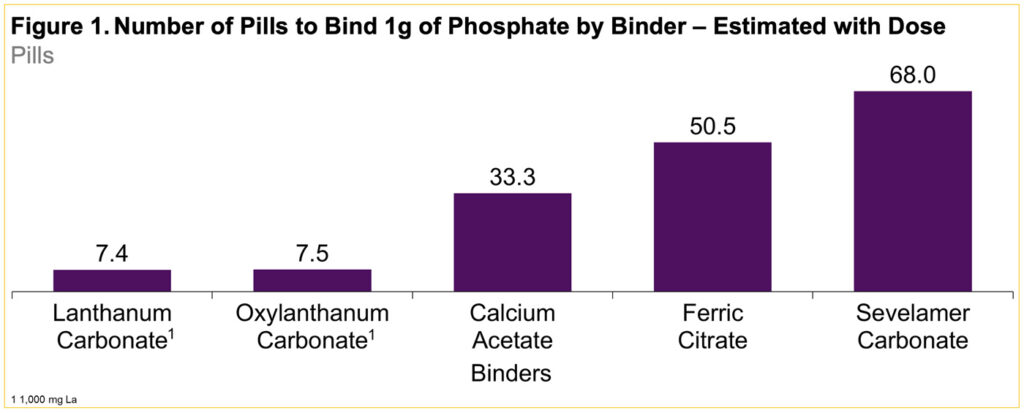
- Each day a high number of phosphate binder pills are typically needed to achieve serum phosphate goals
- Oxylanthanum carbonate (OLC) is a new lanthanum-based nanotechnology product in development with a high in vitro binding capacity as compared to other phosphate binders
- OLC is formulated as a small pill that is swallowed whole instead of being chewed before swallowing as is the case for lanthanum carbonate (Figure 1)
- The safety of OLC has been studied in >100 healthy subjects
- The most common treatment-related AE (>5%) in an OLC bioequivalence (BE) study was nausea

OBJECTIVE
This study aims to evaluate the tolerability of clinically effective (serum phosphate ≤5.5 mg/dL) doses of OLC in patients on hemodialysis
Methods
- This open-label, single-arm, multicenter, multidose study will enroll up to 90 patients on hemodialysis who require phosphate binder therapy and have mean historical serum phosphate levels between ≥4.0 and ≤7.0 mg/dL for ≥8 weeks
- The primary endpoint of the study is tolerability as assessed by the incidence of discontinuations due to treatment-related AEs
- In addition to AEs, the pharmacokinetics of OLC will be evaluated
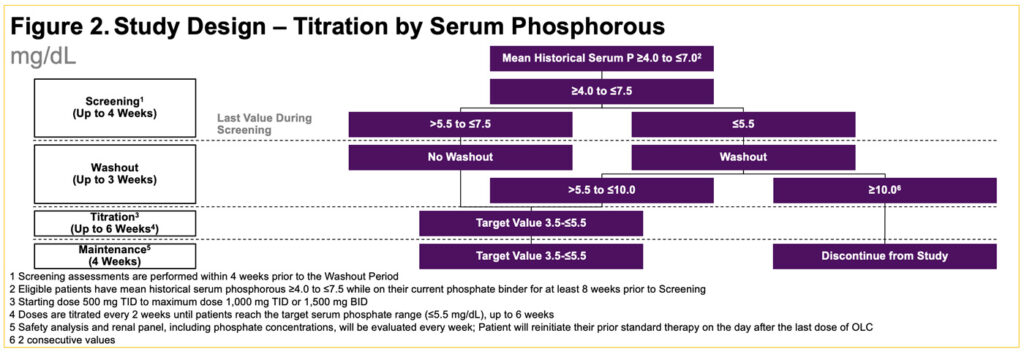
- Participants will undergo up to 3 weeks of phosphate binder washout until their serum phosphate levels reach >5.5 and ≤10.0 mg/dL (Figure 2)
- During the 6-week Titration Period, all patients will receive 1500 mg/day OLC (500 mg TID with meals/snacks) for the initial 2 weeks, with subsequent titration every 2 weeks until achieving a target serum phosphate level (≤5.5 mg/dL) or to a maximum OLC dose of 3000 mg/day
- After titration, patients will enter a 4‑week Maintenance Period at the effective OLC dose
Results
- We intend to present results elucidating tolerability of clinically effective doses of OLC in patients on dialysis

CONCLUSIONS
- The tolerability of OLC at doses effective in achieving a serum phosphate ≤5.5 mg/dL in patients on hemodialysis will be evaluated in this study
References:
1. USRDS. USRDS Annual Data Report. 2020.
2. Wang S. et al., J Ren Nutr. 2014. Mar.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.