
OLC
Redefining phosphate control with oxylanthanum carbonate
Promising potential for patients eager for a solution
Oxylanthanum carbonate (OLC) is a next-generation lanthanum-based phosphate binding agent utilizing proprietary nanoparticle technology being developed for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD) on dialysis. OLC may have meaningful patient adherence benefits over currently available treatment options as it lowers the pill burden for patients in terms of the number and size of pills per dose.
Hyperphosphatemia has been overlooked for far too long
Hyperphosphatemia affects more than 80% of patients on dialysis and has been associated with increased morbidity and mortality.¹ Clinical practice guidelines recommend lowering elevated phosphate levels toward the normal range of 2.5-4.5 mg/dL.²
Representative Sample of US Dialysis Patients
Despite the availability of 7 FDA-approved phosphate-lowering therapies, hyperphosphatemia remains uncontrolled in an estimated 75% of US dialysis patients.³
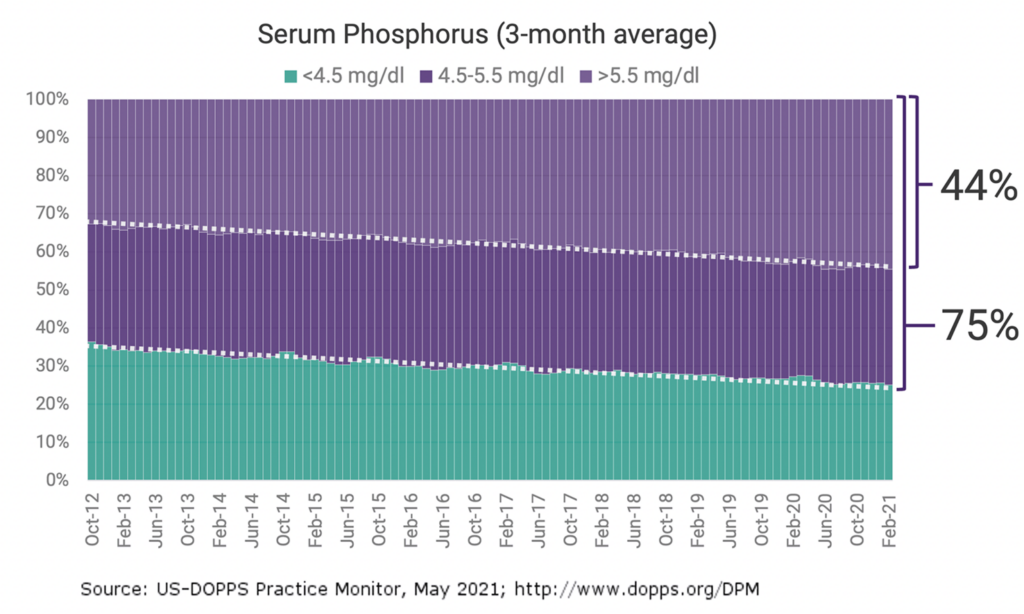
Existing treatments are failing patients
Current phosphate-lowering therapies too often force patients and their physicians to choose between inadequate options. No phosphate-lowering therapy has been able to satisfy 3 critical requirements for successful treatment: potency, low pill burden, and palatability.4
Treatment has been a balancing act and patients remain unable to consistently achieve guideline-established target serum phosphorus levels due to nonadherence.5
“The best phosphate binder is the one the patient will take”
– Sharon M. Moe, MD
Indiana University School of Medicine
Potency
Common phosphate binders have insufficient binding capacity for typical phosphorous intake for hyperphosphatemia patients.6
Pill Burden
Insufficient binding capacity leads to high Pill Burden. The median daily pill burden in dialysis patients is 19 pills–one of the highest reported to date in any chronic disease state.3 Half of this pill burden is from phosphate binders.7
Palatability
Chewable binders are not preferred by most patients due to their chalkiness and metallic taste.8 Other binders are hard to swallow due to their large size.5 The unpalatable nature of these treatments may deter patient adherence.
Care without compromise is close in sight
Through proprietary nanoparticle technology, we’re harnessing the known phosphate binding potency of elemental lanthanum to reduce the number and size of pills that patients must take to control hyperphosphatemia.8
If approved, OLC may offer patients a 4x reduction in daily pill burden (by volume) compared to the most prescribed phosphate binder currently.8

OLC: from unexpected origins to promising potential
By seeing things differently, we’re able to harness innovation from unanticipated places. See how the basis for OLC started with a car battery.
OLC news and updates
References
1. Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16(2):520-528. doi:10.1681/ASN.2004070602 2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) [published correction appears in Kidney Int Suppl (2011). 2017 Dec;7(3):e1. doi: 10.1016/j.kisu.2017.10.001]. Kidney Int Suppl (2011). 2017;7(1):1-59. doi:10.1016/j.kisu.2017.04.001 3. US-DOPPS Practice Monitor, May 2021; http://www.dopps.org/DPM 4. Floege J. Phosphate binders in chronic kidney disease: an updated narrative review of recent data. J Nephrol. 2020 Jun;33(3):497-508. 5. Umeukeje EM, Mixon AS, Cavanaugh KL. Phosphate-control adherence in hemodialysis patients: current perspectives. Patient Prefer Adherence. 2018;12:1175-1191. 6. Huml AM, Sullivan CM, Leon JB, et al. The adequacy of phosphorus binder prescriptions among American hemodialysis patients. Ren Fail. 2012;34(10):1258-63 7. Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009 Jun;4(6):1089-96. 8. Data on file. Unicycive Therapeutics, Inc; 2024.