Guru Reddy1, Pramod Gupta1, Atul Khare1, Shalabh Gupta1
1 Unicycive Therapeutics, Inc.
Background
- Inflammation and reactive oxygen species driven mitochondrial permeability transition pore (mPTP) opening causes mitochondrial dysfunction/swelling and eventual cell death over time
- This is implicated in a wide range of acute diseases including acute kidney disease originating from ischemia reperfusion injury or delayed graft function
- Furthermore, unresolved inflammation exacerbates sustained mPTP opening, evident in chronic kidney diseases
- UNI-494 is a selective mitochondrial ATP-sensitive potassium (KATP) channel activator that binds to the KATP channels, which reverses the mitochondrial dysfunction by closing the mPTP
- As drug-drug interactions (DDI) are usually adverse clinical effects, pre-clinical evaluations of DDI risk are an important part of new drug development

OBJECTIVE
We present results from 3 pre-clinical studies of UNI-494, with the goal of evaluating DDI risk
Methods
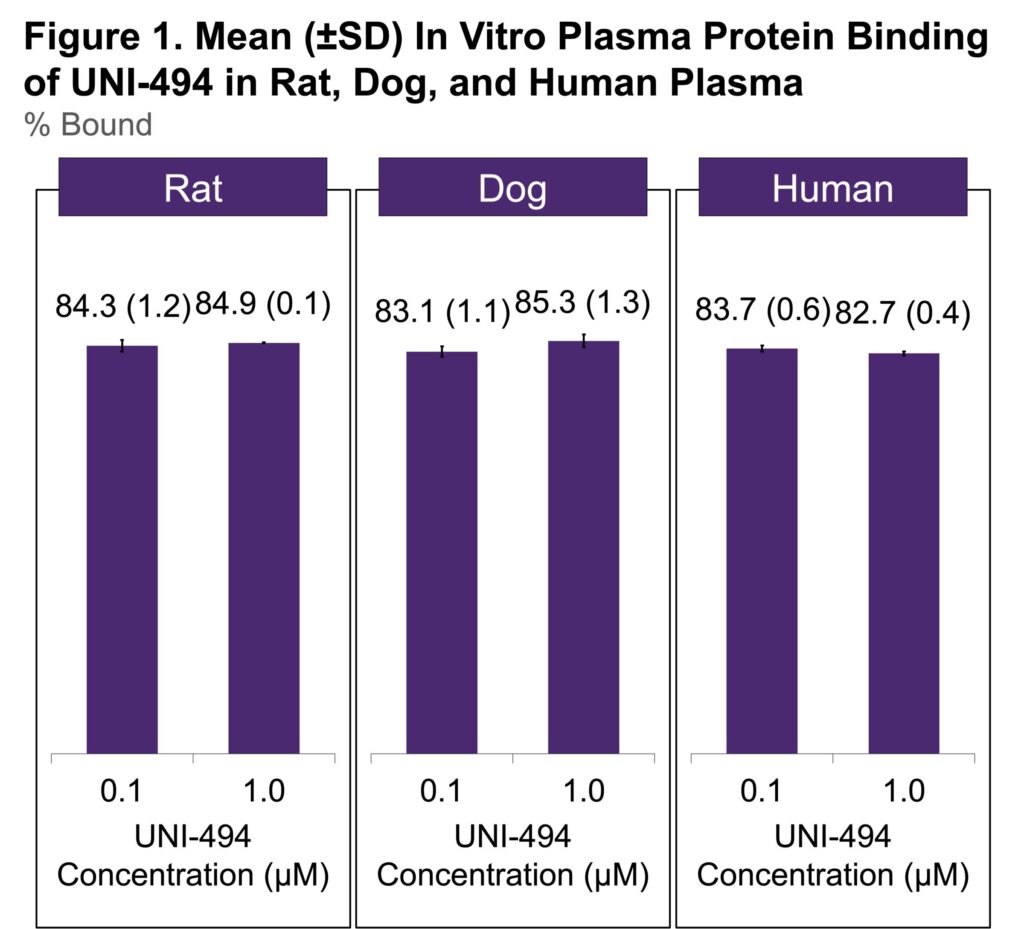
- UNI-494 at concentrations of 0.1 and 1 mM were individually added to the plasma of rats, dogs, and humans, and the in vitro plasma protein binding was determined by the ultrafiltration method
- Each concentration was tested in triplicate
- To evaluate transporter inhibition of P-GP, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1, and MATE2-K, probe substrate transport was measured in the presence and absence of UNI-494
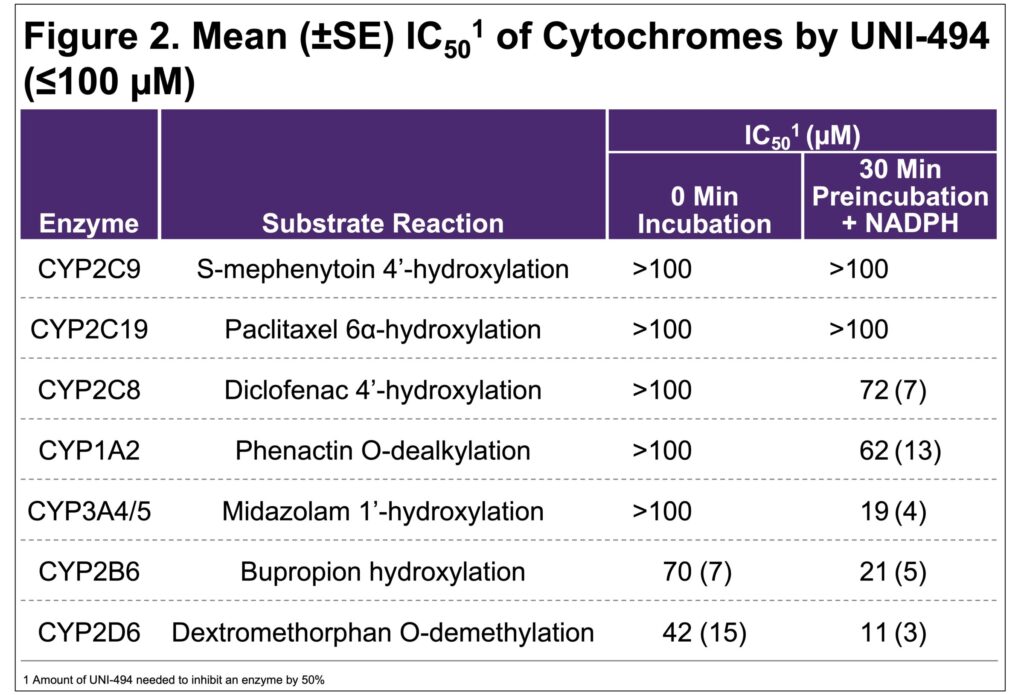
- To evaluate the inhibition of cytochrome P450 enzymes by UNI-494 (<100 mM), automated samples were prepared using a single substrate cocktail incubation with pooled human liver microsomes (0.1 mg/mL), with 0- and 30-minute NADPH pre-incubation and a 5-minute substrate incubation time
- Metabolite formation in the incubation cocktail was analyzed by
- LC-MS/MS, including deuterated internal standards
Results
- The in vitro plasma protein binding ratios of UNI-494 at concentrations of 0.1 mM and 1 mM were 84.3% and 84.9% in rats; 83.1% and 85.3% in dogs; and 83.7% and 82.7% in humans, respectively (Figure 1)
- At IC50 >100 mM, UNI-494 did not inhibit OATP1B1, OATP1B3, or OAT3 and weakly inhibited BCRP and P-glycoprotein
- At IC50 ≤100 mM, UNI-494 showed weak or no inhibition of all CYP450 enzymes tested, with only CYP2D6 approaching moderate inhibition (Figure 2)

CONCLUSIONS
- These results indicate that plasma protein binding for UNI-494 is high and it is not concentration dependent
- UNI-494 had little to no inhibition of the hepatic transporters or CYP enzymes tested
DISCUSSION
- UNI-494 may have a low risk of DDI
- Future studies in patients are warranted
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.