Stuart M. Spraguea Guru Reddyb Douglas Jermasekc Pramod Guptad
aDivision of Nephrology and Hypertension, NorthShore University Health System, Evanston, IL, USA; bPreclinical Research and Development, Unicycive Therapeutics, Inc., Los Altos, CA, USA; cCorporate Strategy, Unicycive Therapeutics, Inc., Los Altos, CA, USA; dPharmaceutical and Business Operations, Unicycive Therapeutics, Inc., Los Altos, CA, USA
Abstract
Background
A key focus for chronic kidney disease management is phosphate control, but currently available binders have suboptimal phosphate-binding capacity, and their characteristics result in low adherence and poor phosphate regulation. Oxylanthanum carbonate, a novel compound that uses proprietary nanoparticle technology to deliver lanthanum, has the potential to combine high phosphate-binding capacity with good intake convenience, thus improving adherence and patient quality of life. The goal of this study was to assess the volume of oxylanthanumCarbonate required to bind 1 g of phosphate and compare it with other currently available phosphate binders to determine which binder allows for the highest normalized potency with the lowest daily medication volume.
Methods
Six phosphate binders were assessed: ferric citrate, calcium acetate, lanthanum carbonate, sevelamer carbonate, sucroferric oxyhydroxide, and oxylanthanum carbonate. Table volume measurements were taken using fluid displacement in corn oil or water. Mean daily dose volume to bind 1 g of phosphate was calculated as volume per tablet multiplied by the mean number of tablets taken per day. Volume to bind 1 g of phosphate was calculated by dividing the volume per tablet by its in vivo binding capacity.
Results
Oxylanthanum carbonate had the lowest mean volume, daily phosphate binder dose volume, and equivalent phosphate-binding dose volume (volume to bind 1 g of phosphate for each binder).
Conclusions
Oxylanthanum carbonate has the lowest daily phosphate binder dose volume and the smallest volume required to bind 1 g of phosphate compared to all other commercially available phosphate binders. A randomized trial that compares gastrointestinal tolerability across binders would be warranted to demonstrate acceptability and adherence in the target population.

Introduction
Chronic kidney disease (CKD) and end-stage kidney disease (ESKD) affect an estimated 37million and 785,000 people in the USA, respectively [1, 2]. For patients with CKD, phosphate regulation (normal phosphate concentrations being between 3 and 4.5 mg/dL) [3] is crucial to preserving the proper function of many biological processes, including energy metabolism, cell signaling, regulation of protein synthesis, skeletal development, and bone integrity [4].
The development of hyperphosphatemia (serum phosphorus concentrations >4.5 mg/dL) is associated with significant pathophysiology and increased mortality risk, including associated elevated risk of cardiovascular disease [5] and an estimated 23% increased mortality risk in CKD patients for each 1 mg/dL increase in serum phosphorus [6]. Almost 75% of patients on dialysis have serum phosphorus concentrations >4.5mg/dL [7]; therefore, a key focus for CKD management is phosphate control.
Phosphate control strategies include restricting dietary phosphate intake, enhancing phosphate elimination with dialysis, and using phosphate binders. Currently, available phosphate binders reduce phosphate absorption by binding to phosphate to form insoluble complexes that are then excreted. Some investigational phosphate control therapies act on one of two phosphate absorption pathways: the active transcellular pathway or the passive paracellular pathway. For example, the novel drug tenapanor blocks paracellular phosphate permeability by inhibiting the sodium/hydrogen exchanger isoform 3 in the GI tract. Tenapanor has been shown to efficiently reduce phosphorus levels in multiple clinical trials [8, 9].
Despite a multifaceted approach, management of phosphatemia remains unsuccessful in many patients [7]. Currently, available binders have suboptimal phosphate-binding capacity, and their characteristics result in low adherence and poor phosphate regulation. Current phosphate binders can only bind about 300 mg phosphate/day, which is not nearly enough to match a daily dietary phosphorus load of about 1,400 mg [8]. Additionally, phosphate binders are the single largest contributor to the daily pill burden in patients on dialysis, accounting for ~50% of total daily pills [10]. Low adherence is attributed to pharmacological characteristics including perceived side effects and intake inconveniences, such as taste, number of pills, and having to take pills with meals [11]. As a result, a study of 135 hemodialysis patients found that 78% of patients were not adherent over 8 weeks, leading to mean phosphatemia levels that were 0.6 mg/dL higher among nonadherent patients compared to adherent patients [11]. Consequently, identifying a phosphate binder that combines high phosphate-binding capacity with good intake convenience may improve adherence and patient quality of life, thereby potentially improving phosphate control and clinical outcomes.
One potential compound is the investigational phosphate binder oxylanthanum carbonate, which is distinct from the approved phosphate binder lanthanumcarbonate in that it uses novel nanoparticle technology. The goal of this study was to assess the volume of oxylanthanum carbonate required to bind 1 g of phosphate and compare it with other currently available phosphate binders to determine which binder allows for the highest normalized potency with the lowest daily medication volume.
Materials and Methods
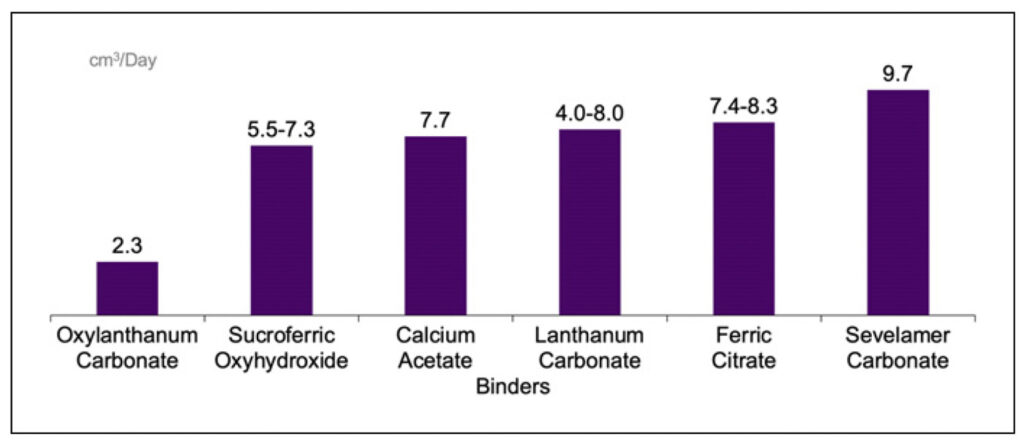
Six phosphate binders were assessed: ferric citrate (1,000 mg), calcium acetate (667 mg), lanthanum carbonate (500 and 1,000 mg lanthanum), sevelamer carbonate (800 mg), sucroferric oxyhydroxide (2,500 mg), and oxylanthanum carbonate (500 and 1,000 mg lanthanum). The table volume measurements were conducted by following the principle of fluid displacement, where the displaced volume was equal to the volume of the tablets immersed in the fluid. Graduated measuring cylinders of 10, 25, 50, or 100 mL were used, depending on the size of the tablet. The medium for volume measurement was corn oil or water. Corn oil was chosen as the tablets remain intact in this medium, and the color of the oil allows volume marks to be easily read. For some tablets, when effervescence/ bubbling was observed in corn oil, water (with added color for easier reading of volume marks) was used. Tablets were slowly dropped with forceps into graduated measuring cylinders containing a fixed volume of suitable liquid, and the volume raised was observed and noted. Each measurement was performed in duplicate.
The mean daily dose volume was calculated as the volume pertablet multiplied by the mean number of tablets taken per day, based on the literature. To calculate the volume to bind 1 g of phosphate for approved phosphate binders, the volume per tablet was divided by its in vivo binding capacity (grams of phosphate bound by the amount of active ingredient in 1 tablet) based on the NIH DailyMed website [12]. The phosphate-binding capacity data for the investigational phosphate binder oxylanthanum carbonate were obtained from an in vitro study.
Results
The volumes by tablet are listed in Table 1. The lowest mean volume was demonstrated with oxylanthanum carbonate (500 mg lanthanum), followed by oxylanthanum carbonate (1,000 mg lanthanum), calcium acetate (667 mg), ferric citrate (1,000 mg), sevelamer carbonate (800 mg), lanthanum carbonate (500 mg lanthanum), sucroferric oxyhydroxide (2,500 mg), and lanthanum carbonate (1,000 mg lanthanum). The daily phosphate binder dose volume was lowest with oxylanthanum carbonate and highest with sevelamer carbonate, at 2.3 and 9.7 cm3, respectively (shown in Fig. 1). The equivalent phosphate-binding dose volumes – the volume to bind 1 g of phosphate for each binder – were 5.6, 19.8, 25.0, 46.5, and 73.4 cm3 for oxylanthanum carbonate, lanthanum carbonate, calcium acetate, ferric citrate, and sevelamer carbonate, respectively.
Table 1. Phosphate binder volumes by tablet
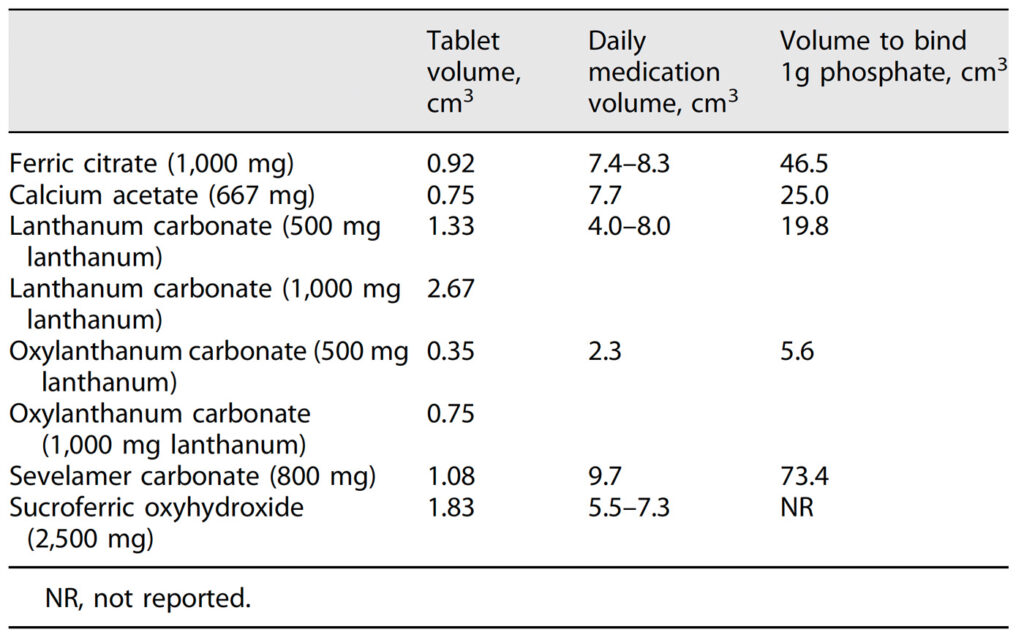
Fig. 1. Daily medication volumes. The daily dose volume of oxylanthanum carbonate was 3- to 4-fold lower than other phosphate binders

DISCUSSION
To the best of our knowledge, this is the first publication to report daily medication volumes and the medication volume required to bind 1 g of phosphate across phosphate binders. The novel phosphate binder oxylanthanum carbonate had a significantly lower daily dose volume compared to currently available binders (2.3 vs. 4.0–9.7 cm3) and had the greatest phosphate-binding “potency,” as demonstrated by the comparatively low volume required to bind 1 g of phosphate (5.6 vs. 19.8–73.4 cm3). These results suggest that oxylanthanum carbonate will be much easier for patients to ingest and may be particularly valuable for patients with difficult-to-control phosphate.
The ultimate goal of phosphate management strategies for patients with CKD or ESKD is to achieve normal phosphate levels (3–4.5 mg/dL) [3] as per KDIGO recommendations [13]. However, the large proportion of patients on dialysis with phosphate concentrations over 5.5 mg/dL [7], despite treatment with phosphate binders, highlights the current deficiencies in phosphate management. Suboptimal phosphate control may result from low phosphate-binding capacity and poor adherence to phosphate binders. Binders can only control about 10–25% of the average daily dietary phosphorus intake [14]. In addition, almost 80% of patients on dialysis are not adherent to the prescribed dosing schedule, which negatively impacts their phosphorus concentrations [11]. Given that a higher daily medication burden is associated with significantly lower adherence and higher serum phosphorus levels [10], it is then logical to assume that a binder with increased phosphate-binding capacity and a lower daily medication burden would enhance adherence and consequently improve phosphate control and clinical outcomes.
Of note, other factors also constitute important barriers to phosphate binder adherence, including the burden of taking daily medication, high dosing frequency (e.g., 3 times/day), adverse side effects, tablets that are hard to swallow (due to taste, shape, or size), lack of belief in the effectiveness of phosphate binders, and lack of knowledge about how phosphate binders work and why taking them consistently per prescribing information is important [11]. Improving one ormore of these factorsmay increase adherence, thereby increasing phosphate binder efficacy.
There are some limitations to this study. First, the calculations performed in this study were based on the literature reporting the mean number of phosphate binder tablets taken per day, which may not be generalizable to the target population of patients with CKD and ESKD. However, these study results are valuable because they provide the first published comparison of medication volumes across binders and can serve as a guide for clinicians looking to select a therapy that will improve ease of ingestion. Second, this study did not assess the tolerability of phosphate binders and gastrointestinal adverse events, another common reason for binder nonadherence or discontinuation [11]. However, oxylanthanum carbonate was safe and well tolerated in preclinical studies and a phase 1 trial in healthy volunteers [15].
Oxylanthanum carbonate combines high phosphatebinding capacity with a low medication volume, making this a new binder option that could potentially improve adherence and clinical outcomes. For clinicians, improved phosphate control will allow them to focus patient care on other aspects of CKD management to optimize patient quality of life and reduce morbidity and mortality.
CONCLUSIONS
We demonstrated that oxylanthanum carbonate, a novel compound that uses proprietary nanoparticle technology to deliver lanthanum, has the lowest daily phosphate binder dose volume and the smallest volume required to bind 1 g of phosphate compared to all other commercially available phosphate binders. A randomized trial that compares gastrointestinal tolerability across binders would be warranted to demonstrate acceptability and adherence in the target population.
Statement of Ethics
An ethics statement was not required for this study type; no human or animal subjects or materials were used.
Conflict of Interest Statement
Dr. Sprague reports grants and personal fees from Ardelyx, grants from Amgen, grants and personal fees from OPKO Pharm, and grants and personal fees from Vifor. Pramod Gupta, Guru Reddy, and Douglas Jermasek are employees of Unicycive Therapeutics, Inc.
Funding Sources
The study was funded by Unicycive Therapeutics, Inc.
Author Contributions
Stuart Sprague: Conceptualization, data analysis, and writingv(original draft, review, and editing).
Pramod Gupta: Conceptualization, study design, data acquisition, data analysis, and writing (review and editing).
Guru Reddy: Conceptualization, study design, data acquisition, data analysis, and writing (review and editing).
Doug Jermasek: Conceptualization, study design, and writing (review, and editing).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References:
- Chronic kidney disease in the United States. Atlanta, GA: Centers for Disease Control and Prevention, Services UDo-HaH; 2021.
- 2020 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: United States renal data system. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020.
- Suki WN, Moore LW. Phosphorus regulation in chronic kidney disease. Methodist Debakey Cardiovasc J. 2016;12(4 Suppll):6–9.
- Goretti Penido M, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27(11):2039–48.
- Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74(2):148–57.
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16(2):520–8.
- Serum phosphorus (most recent), categories. DOPPS Practice Monitor; 2022 Available from: https://www.dopps.org/DPM-HD/Files/phosphmgdl_c_overallTAB.htm.
- Pergola PE, Rosenbaum DP, Yang Y, Chertow GM. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY). J Am Soc Nephrol. 2021;32(6):1465–73.
- Block GA, Rosenbaum DP, Yan A, Chertow GM. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30(4):641–52.
- Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96.
- Van Camp YP, Vrijens B, Abraham I, Van Rompaey B, Elseviers MM. Adherence to phosphate binders in hemodialysis patients: prevalence and determinants. J Nephrol. 2014;27(6):673–9.
- Medicine NNLo. Daily Med [cited 2022 September 27]. Available from: https://dailymed.nlm.nih.gov/.
- Kidney Disease: Improving Global Outcomes KDIGO CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKDMBD). Kidney Int Suppl. 2017;7(1):1–59.
- McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001-2014. Nutrients. 2017; 9(2):95.
- Study to assess the safety and the phosphate binding capacity of renazorb (SPI-014) [cited 2022 September 28]. Available from: https://clinicaltrials.gov/ct2/show/NCT01560884.