S. MEDICHERLA1, G. REDDY1, P. GUPTA1, S. GUPTA1
1 Unicycive Therapeutics, Inc.
Background
- End-stage renal disease (ESRD) affects >7M people worldwide1 and ~70% of patients with ESRD have hyperphosphatemia2
- Current hyperphosphatemia treatment options are dietary phosphate (P) restriction, dialysis, and P binders. However, not only are these hyperphosphatemia treatment options not highly effective for P control, they also negatively impact patient quality of life
- Thus, there is an unmet need for novel therapeutic innovations that maintain efficacy while reducing the required number of tablets and adverse effects, thereby increasing adherence and potentially improving clinical outcomes
- Tenapanor is a sodium hydrogen exchanger inhibitor used to reduce serum P in adults with chronic kidney disease (CKD) on dialysis as an add-on therapy with P binders3. Tenapanor has a unique mechanism of action that blocks paracellular absorption, thus reducing intestinal P absorption
- Combination treatments, especially those employing therapies with distinct mechanisms of action, may improve P control while reducing the negative characteristics of current P binders
- A previous study conducted by King et al.4 found that when administered together, tenapanor and sevelamer decreased urinary P excretion significantly more than either tenapanor or sevelamer alone across all sevelamer dose levels
- Oxylanthanum carbonate (OLC) is a novel nanotechnology product that combines lanthanum, which has highest binding capacity vs other P binders, with smaller pill size that is swallowed with water vs chewed5

OBJECTIVE
The objective of our study was to evaluate the effects of tenapanor and OLC on P excretion in rats on a high phosphorus diet
Methods
- In our study, we utilized the study design and dosage regimen from King et al.4 involving sevelamer and tenapanor
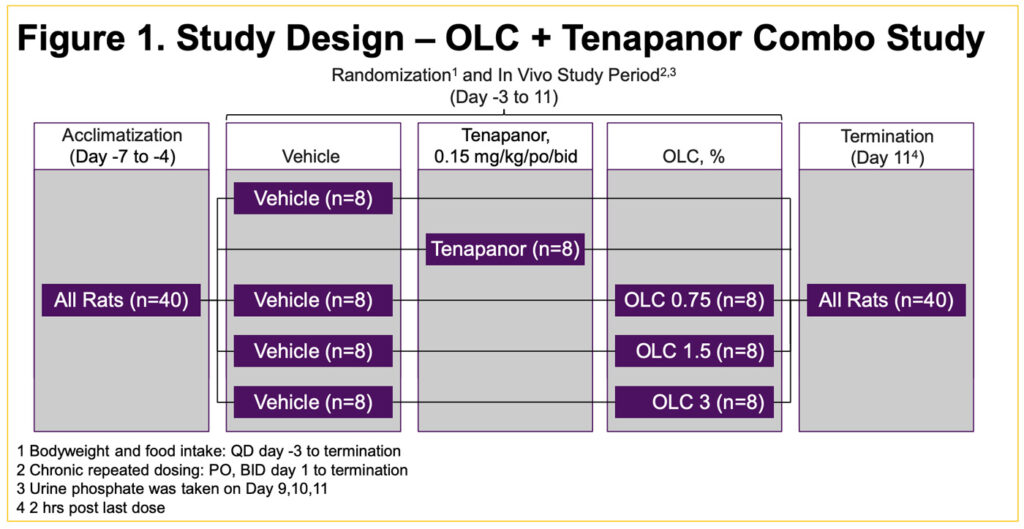
- The study consisted of acclimatization, randomization, in vivo study period, and termination (Figure 1)
- 40 male Sprague Dawley rats fed standard chow 1 week prior to study start and then spiked with an additional 0.4% inorganic phosphate [1:1 sodium:potassium salt, 1.1% (wt/wt) total phosphate content] for rest of the study4
- On study Day -1, rats were randomized into study groups (n = 8): 1) Vehicle, 2) tenapanor 0.15 mg/kg, 3) Vehicle + OLC 0.75%, 4) Vehicle + OLC 1.5%,
- 5) Vehicle + OLC 3% (Figure 1)
- From 11 days, Vehicle and tenapanor were dosed orally (PO) twice/day via oral gavage whereas OLC was incorporated into the diets
- Food intake was measured daily from study Day -3 until study Day 11, when the animals were euthanized
- 24-hour urine samples were collected using metabolic cages on Days 9, 10, and 11 for urinary P measurements
- Mean urine P excretion concentrations for each animal from Days 9 to 11 were averaged by treatment group
Results
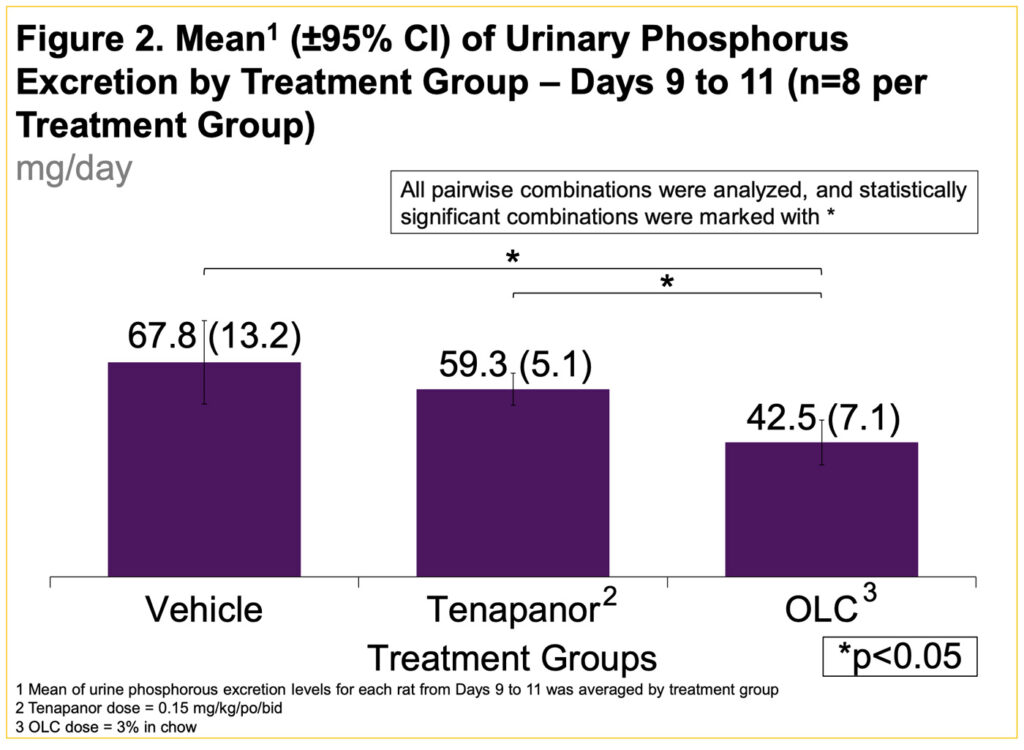
- Reduction of urinary P excretion with tenapanor was not significantly different compared to Vehicle (Figure 2)
- Compared to Vehicle alone (group 1), tenapanor demonstrated 8.5 mg/day less urinary P excretion while OLC demonstrated 25.3 mg/day less urinary P excretion, a significantly greater reduction in urinary P excretion with OLC compared to tenapanor (p<0.05) (Figure 2)

CONCLUSIONS
- Tenapanor did not significantly reduce urinary P excretion
- OLC demonstrated 3x more effective urine P reduction vs tenapanor
- Subsequent analyses will focus on examining the combination of tenapanor and OLC
- OLC and tenapanor utilize 2 different mechanisms of action to manage P levels. The combination may lead to synergistic effects on lowering P levels while providing patients with the benefit of reduced pill burden
- Future studies should evaluate tenapanor + OLC in humans with CKD
References:
1.Lv JC, et al., Adv Exp Med Biol. 2019. Aug.
2.K/DOQI clinical practice guidelines. Am J Kidney Dis. 2003. Oct.
3.XPHOZAH (tenapanor) tablets, for oral use [prescribing information]. Ardelyx. 2023.
4.King AJ, et al., Am J Physiol Renal Physiol. 2021. Jan. Arenas MD, et al., Nefrologia. 2010. May.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.