Geoff A. Block1, MD; Glenn M. Chertow2, MD, MPH; Guru Reddy3, PhD; Sanjay Mourya3; Martha Block1; Steve J. Hasal3, PhD; Shalabh Gupta3, MD; Pablo E. Pergola4, MD, PhD
1US Renal Care, San Antonio, TX, 78211; 2Stanford University School of Medicine, Stanford, CA, 94305; 3Unicycive Therapeutics, Inc., Los Altos, CA, 94022; 4Renal Associates, PA, San Antonio, TX, 78212
BACKGROUND
- In healthy individuals, serum phosphate (sP) concentrations are maintained within a relatively narrow range
- Renal tubular reabsorption of phosphate is reduced in patients with impaired kidney function
- Three primary phosphate control strategies are available – restricting dietary phosphate intake, enhancing phosphate elimination through more frequent or longer duration hemodialysis sessions, and using oral phosphate lowering therapy
- Oxylanthanum carbonate (OLC) is a novel compound being developed for the treatment of hyperphosphatemia in patients with ESRD that utilizes proprietary nanoparticle technology

STUDY PURPOSE
We conducted this study to assess the tolerability of and safety of OLC at doses that achieve satisfactory control of sP (≤5.5 mg/dL) in patients with CKD on hemodialysis receiving maintenance therapy for hyperphosphatemia
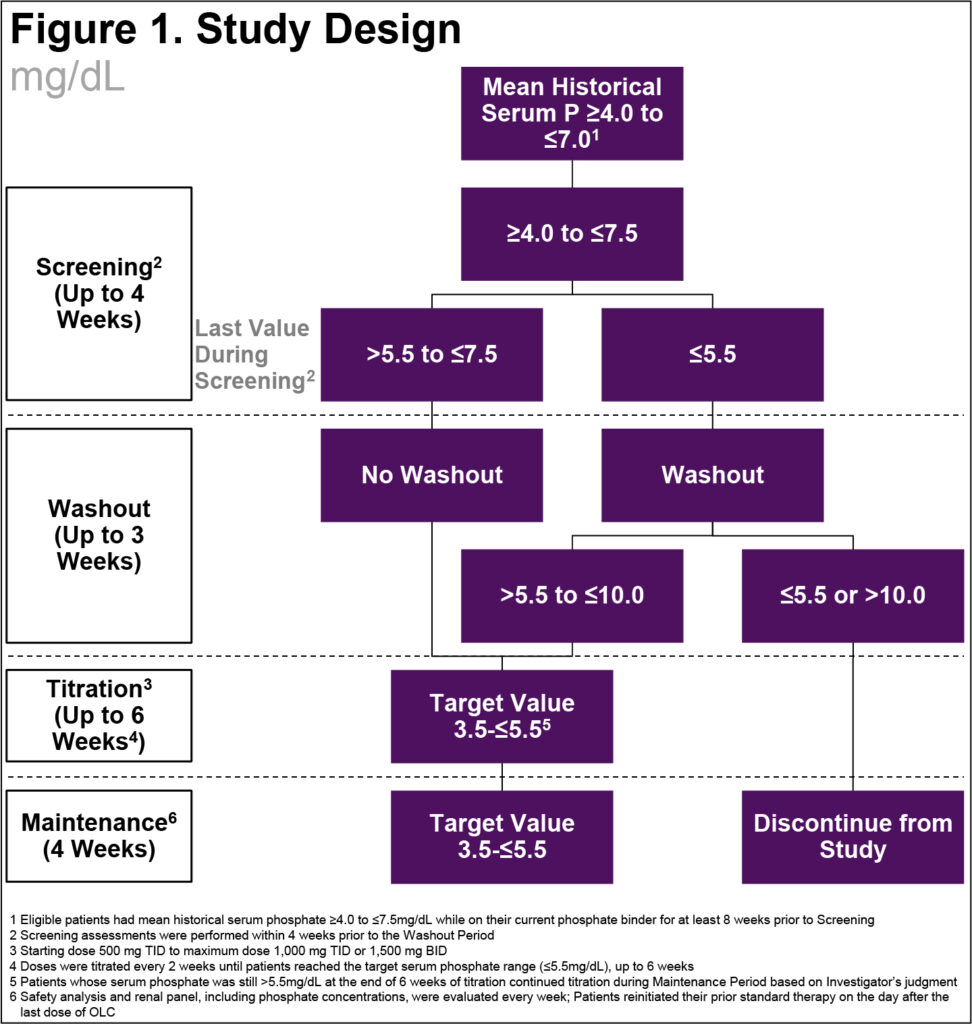
Study Design
- This was a Phase 2, open-label, single-arm, multicenter, multidose study in adult patients with CKD with hyperphosphatemia receiving maintenance hemodialysis
- After an up to 4-week Screening Period, the trial was divided into three parts: a Washout Period (1 to 3 weeks), a 6-week, open-label, Dose-Titration Period, and a 4-week, open-label, Maintenance Period (Figure 1)
- The study was designed to enroll approximately 90 patients to have 60 evaluable patients who entered the Maintenance Period; the number of patients was not based on statistical assumptions
- Once weekly, we assessed tolerability based on the incidence of discontinuations due to treatment related adverse events (AEs)/adverse drug reactions (ADRs) and laboratory panel, including sP, was performed. At the end of the study, patients reinitiated their prior phosphate binder therapy
- We defined baseline as the last measurement prior to the first dose of study drug
- We assessed safety based on reported/elicited AEs, clinical laboratory assessments, vital signs, 12-lead electrocardiograms, and physical examinations
- Patients were considered evaluable if their sP was ≤5.5 mg/dL at the end of titration and received at least one dose of OLC in maintenance
Results
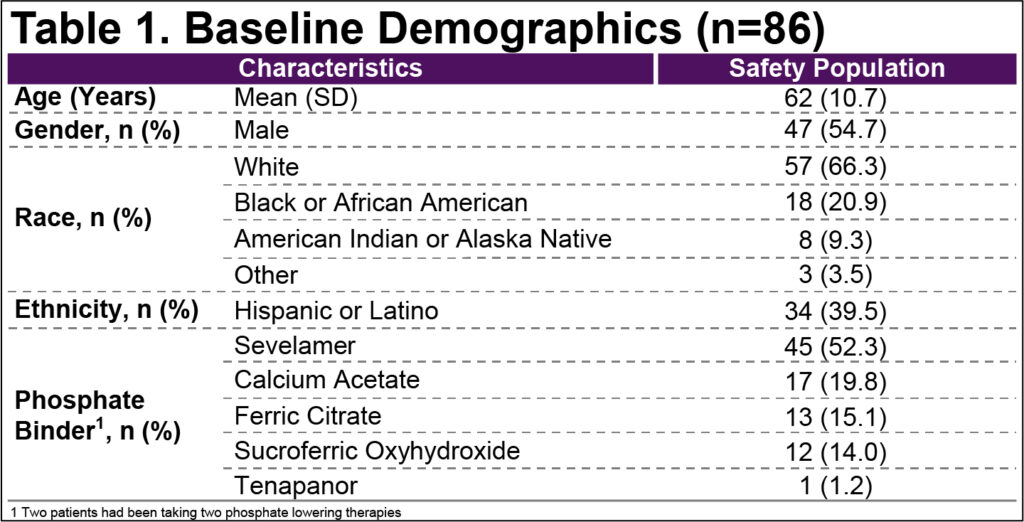
- Baseline demographics were typical of the target population (Table 1)
- 128 patients were screened, and 86 patients received ≥1 dose of OLC in titration. 78 patients entered Maintenance of whom 71 were evaluable and 7 were not evaluable but followed for safety
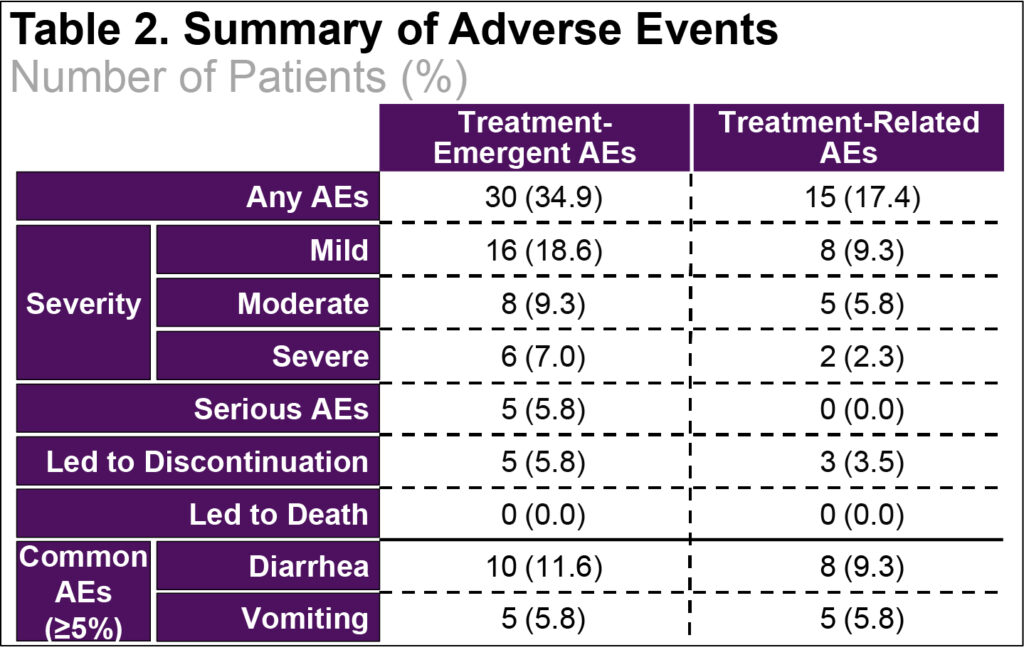
- Most TEAEs were mild to moderate in severity (Table 2)
- There were no deaths. Five patients with SAEs, but none were assessed as related to OLC and 5 patients discontinued due to AEs, but only 3 were due to ADRs (Table 2)
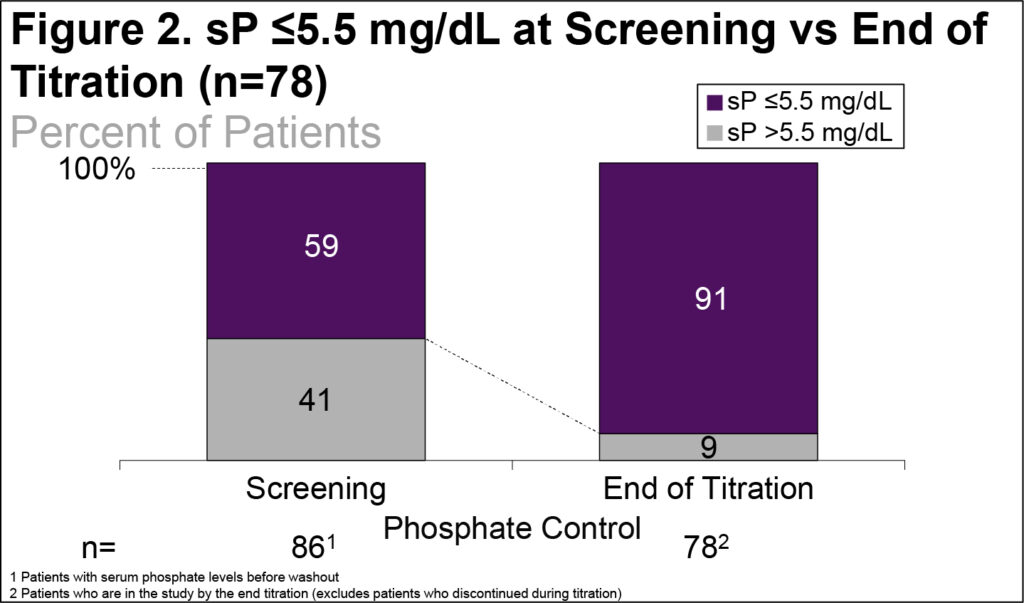
- The percent of patients with sP ≤5.5 mg/dL increased from 59% at Screening to 91% at the end of titration (Figure 2)
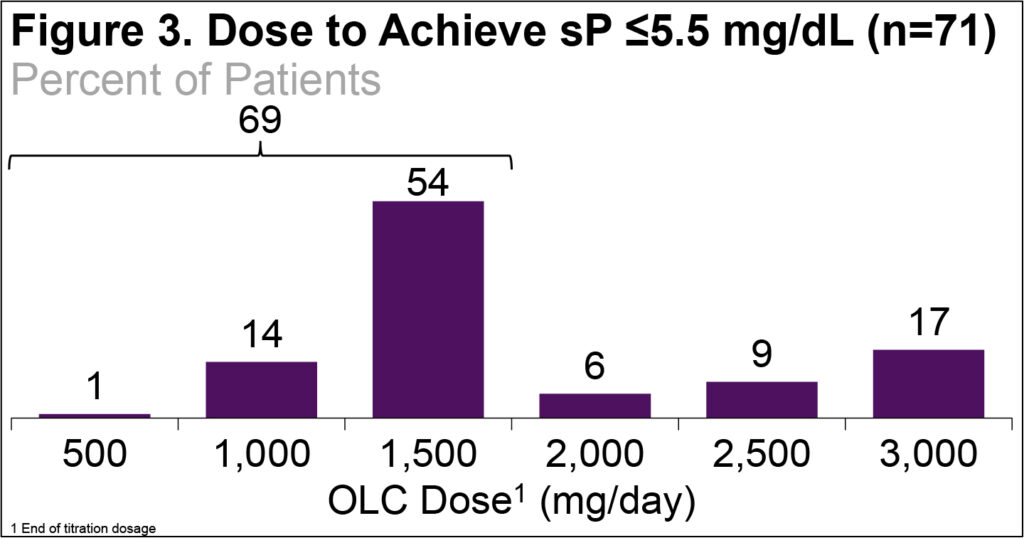
- Most patients (69%) who achieved the target sP did so with ≤1500 mg/day (Figure 3)

Conclusions
- OLC was safe and well-tolerated with ADRs commonly seen in this patient population and with other phosphate binders
- Use of OLC enabled adequate control of sP in >90% of patients who entered Maintenance
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.