G. REDDY1, S. MOURYA1, S. HASAL1, S. GUPTA1
1 Unicycive Therapeutics, Inc.
Background
- Acute kidney injury (AKI) is a clinical syndrome defined by the sudden loss of kidney function1, resulting in an inability to maintain electrolyte, acid-base and water balance2,3
- Currently, there are no effective treatments for AKI approved by the US Food and Drug Administration or the European Medicines Agency (EMA); management of the condition is primarily supportive2,4
- The most common cause of AKI was thought to be ischemia3
- However, more recently the focus has shifted from clinical causes to the underlying cellular processes inherent in these situations, in particular the dysfunction of mitochondria in AKI4
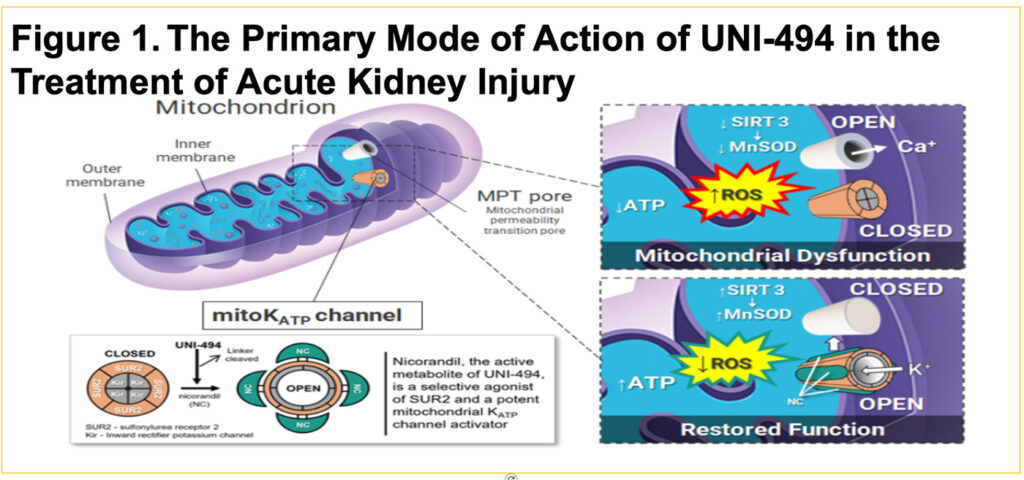
- Inflammation and reactive oxygen species (ROS) driven mPTP opening causes mitochondrial dysfunction/swelling and eventual cell death over time
- This is implicated in a wide range of acute diseases including acute kidney disease (AKI) originating from ischemia reperfusion injury (IRI) or delayed graft function (DGF)
- Furthermore, unresolved inflammation exacerbates sustained mPTP opening, evident in chronic kidney diseases (CKD)
- Nicotinamide adenine dinucleotide (NAD+) can suppress the frequency and duration of mPTP opening
- UNI-494 is a novel nicotinamide ester derivative and selective mitochondrial ATP-sensitive potassium channel activator that binds to the ATP-sensitive potassium (KATP) channels, which reverses the mitochondrial dysfunction by closing mPTP pore (Figure 1)

OBJECTIVE
This study aims to evaluate the safety, tolerability, and pharmacokinetics (PK) of UNI-494
Methods
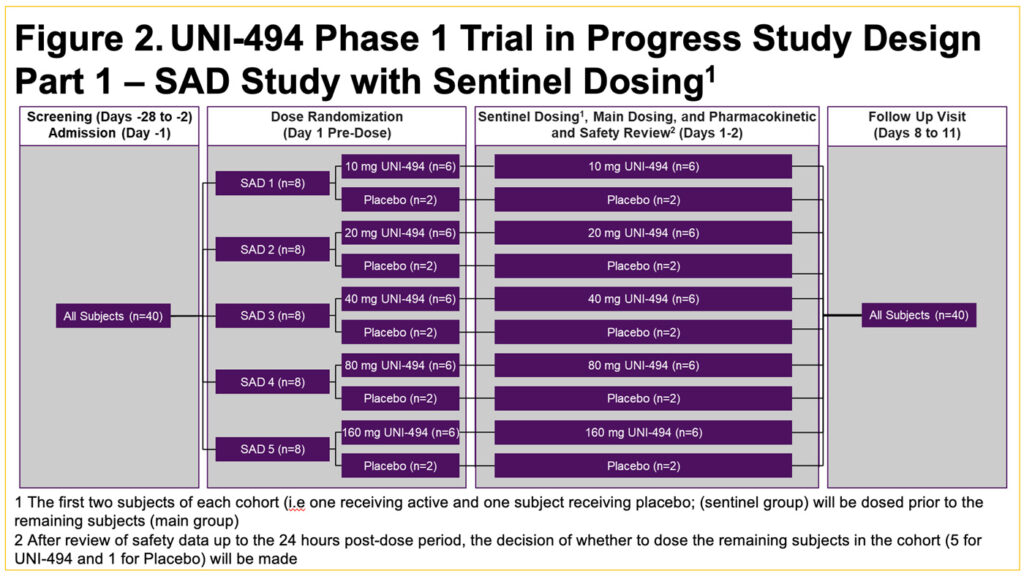
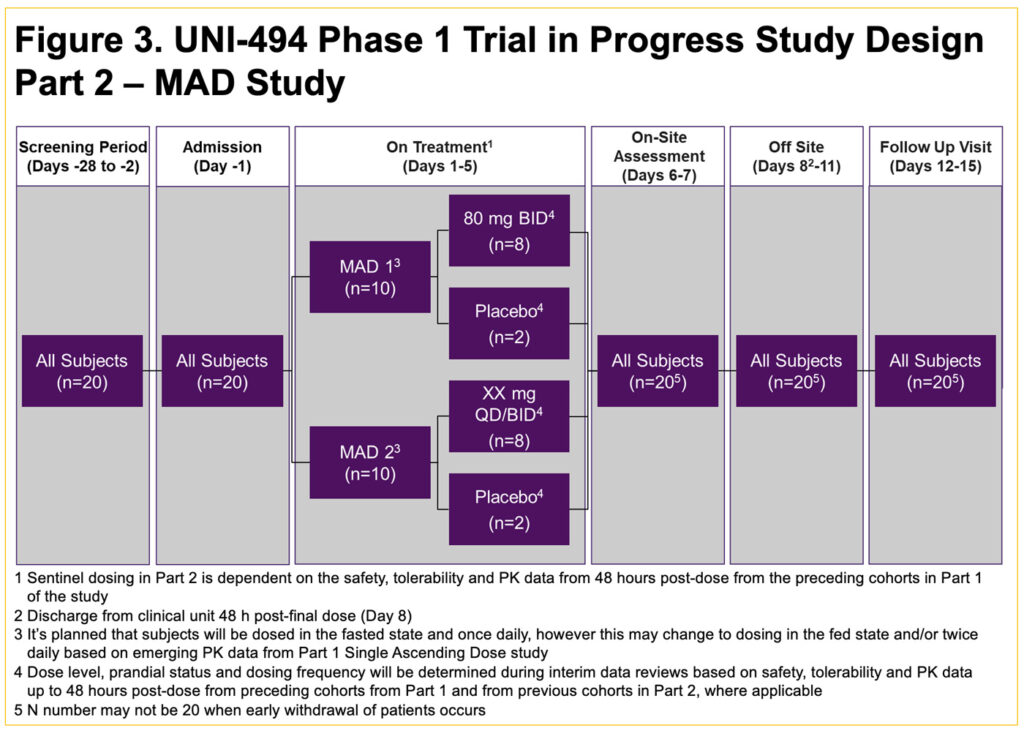
- This is a single-center, double-blind, placebo-controlled, randomized single ascending dose (SAD) (Part 1) (Figure 2) and multiple ascending dose (MAD) (Part 2) (Figure 3) study in healthy male subjects and female subjects of non-childbearing potential
- Part 1 will enroll up to approximately 40 subjects in 5 cohorts of 8 subjects each (randomized to a ratio of 6 active and 2 placebo per cohort) (Figure 2)
- Main inclusion criteria were healthy males and WONCBP, aged 18 to 55 inclusive at the time of signing informed consent
- Body mass index (BMI) of 18.0 to 32.0 kg/m2 as measured at screening
- There will be an interim decision meeting after each cohort/period, to review the safety, tolerability, and pharmacokinetics (PK) data up to 48 hours post-dose in order to decide the dose level for the subsequent cohort
- Part 2 will enroll approximately 20 subjects in 2 cohorts of 10 subjects each, randomized to a ratio of 8 active treatment to 2 placebo who will be dosed for 5 days (Figure 3)
- The dose level for the Part 2 Cohort 1 (80 mg BID) was selected based on the safety, tolerability, and PK data from Part 1
- Pharmacokinetic assessments included collecting blood samples for PK analysis at regular time intervals, and analysis of the plasma concentration-time data for UNI-494, nicorandil and 1-cyclohexylethylamine
- The safety assessments included adverse events monitoring, ECG, vital signs, clinical laboratory tests (clinical chemistry, hematology, and urinalysis), and physical examinations
Results
- We intend to present results elucidating safety, tolerability, and pharmacokinetics of UNI-494 in healthy subjects by 2H 2024

CONCLUSIONS
- The safety, tolerability, and pharmacokinetics of UNI-494 in healthy subjects will be evaluated in this study
References:
1. Kellum JA. et al., Nat Rev Dis Primers. 2021. July.
2.Mercado MG. et al., Am Fam Physician. 2019. Dec.
3.Makris K. et al., Clin Biochem Rev. 2016. May.
4.Szeto HH, J Am Soc Nephrol. 2017. Oct.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive Therapeutics, Inc.