Vandana Mathur1, MD, FASN; Steve Hasal2, PhD; Guru Reddy2, PhD; Shalabh Gupta2, MD
1Mathur Consulting, LLC; 2Unicycive Therapeutics, Inc.
Background
- End-stage renal disease (ESRD) affects more than 7 million people worldwide1 and approximately 70% of patients with ESRD have hyperphosphatemia2
- In patients with CKD G3a through G5D with hyperphosphatemia, current Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend treatments such as dietary intervention, phosphate binders, and dialysis to lower elevated serum phosphate concentrations toward the normal range while avoiding hypercalcemia (calcium levels above 10.2 mg/dL [>2.55 mmol/L])3
- Phosphate binders (PB) are integral to the management of hyperphosphatemia in patients with end-stage kidney disease. Effectiveness of PBs is adversely affected by non-adherence and limitations of phosphate-binding capacity relative to dietary intake
- Lanthanum carbonate (LC): approved PB; must be chewed completely
- Oxylanthanum carbonate (OLC): oral tablet phosphate binder designed to be swallowed whole as opposed to being chewed
- For equivalent-phosphate-binding dose volumes of 1000 mg, referred to as the active moiety, oxylanthanum carbonate demonstrates a significant reduction in pill volume when contrasted with LC, measuring 2.3 cm^3 for the former and 8.0 cm^3 for the latter. Thus, formulations with smaller pill sizes are possible4

OBJECTIVE
Demonstrate the pharmacodynamic (PD) equivalence of orally administered OLC 1000 mg TID to LC 1000 mg TID in healthy subjects
Methods
- Phase 1, single-center, randomized, open-label, controlled, 2-way crossover study in healthy volunteers designed to establish the pharmacodynamic equivalence between OLC swallowable tablets 1000mg TID and LC chewable tablets 1000 mg TID
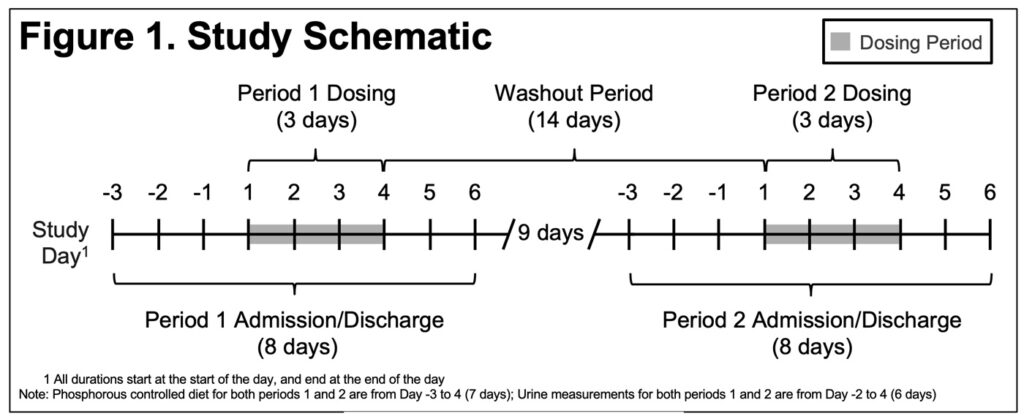
- The study consisted of a screening period (28 days before Day -4 of Period 1), 2 dosing periods separated by a 14-day washout period, and a follow-up period 7 days after last study drug dose(Figure 1)
- Study subjects were housed in an inpatient clinical research unit from Days -3 to 5 of each treatment period
- Randomization was 1:1 to order of treatment (i.e., OLC/LC or LC/OLC)
- Each treatment period included a 2-day Baseline (BL) Period (Days -2 and -1) during which the 24-hour baseline urine P was established and a 3-day Evaluation Period (Days 1 to 3)
- Subjects receive one 1000 mg tablet TID of OLC (swallowed whole) or LC (chewed) after each of 3 meals on Days 1 to 3
- All subjects received the same standardized meals, controlled for daily P (1400 mg/day) during the in-unit period, starting at Day -3
- 24-hour urine samples were collected for 5 days beginning Day -2
- Primary outcome variable was Least Squares (LS) mean change in daily urinary P excretion from BL (48 hrs prior to dosing) to Evaluation Period (Days 1-3)
- Primary pharmacodynamic variable was assessed using a mixed-effect linear model with fixed effects for sequence, period, and study drugs; random effect for individual within-sequence & baseline as a covariate
- Based on the mixed-effect linear model, a standard 90% CI was constructed for the difference in LSM of change in urinary phosphorous excretion from the Baseline Period to the Evaluation Period
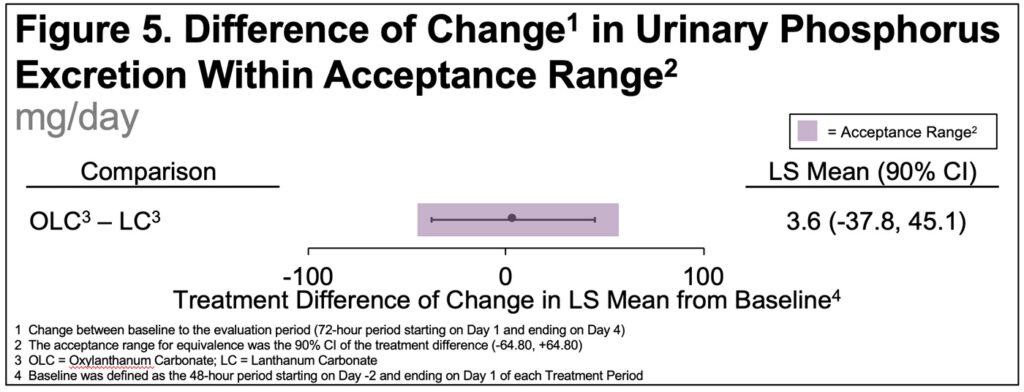
- In addition, a reference interval (acceptance range) for the treatment (effect) was defined as ±20% of the LSM of the primary pharmacodynamic variable for LC
Results
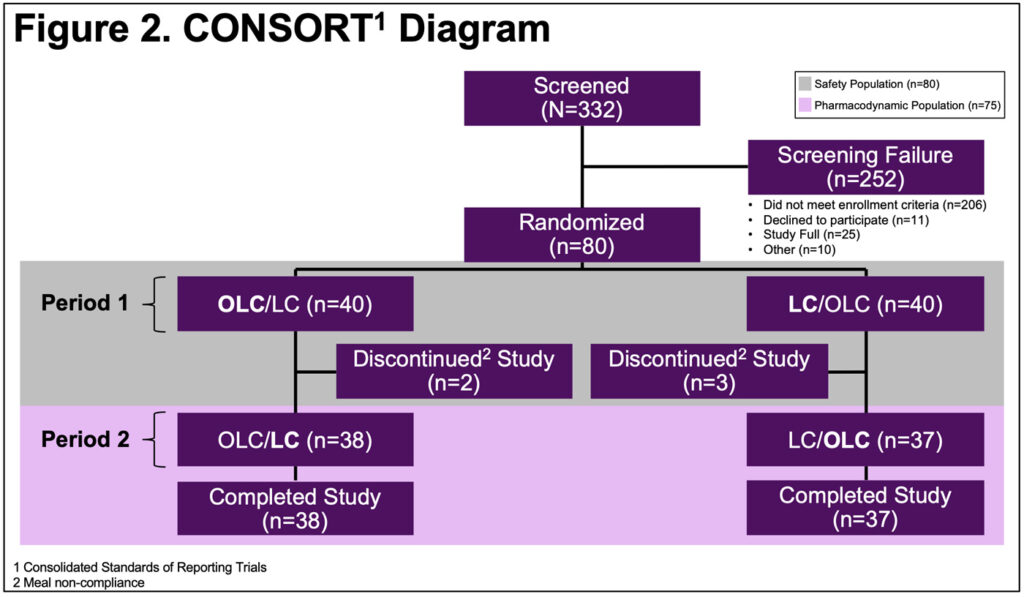
- 75 of 80 randomized subjects were analyzed for the primary (equivalence) endpoint(Figure 2)
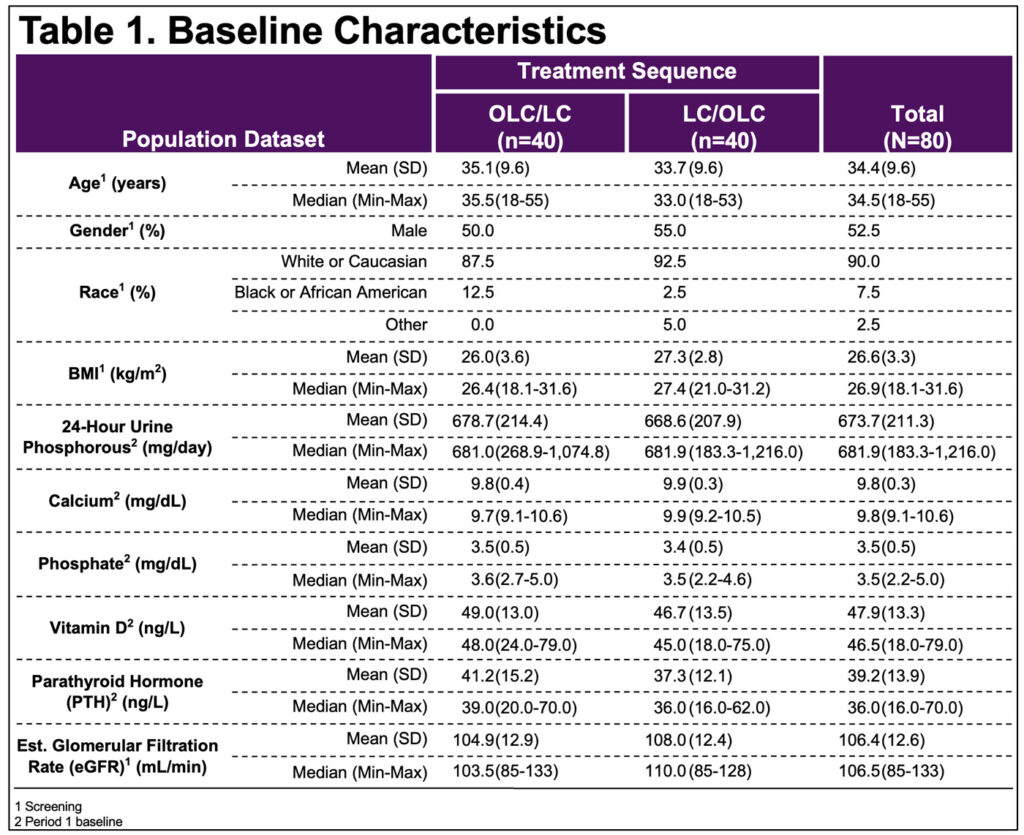
- BL characteristics balanced between OLC/LC & LC/OLC sequences(Table 1)
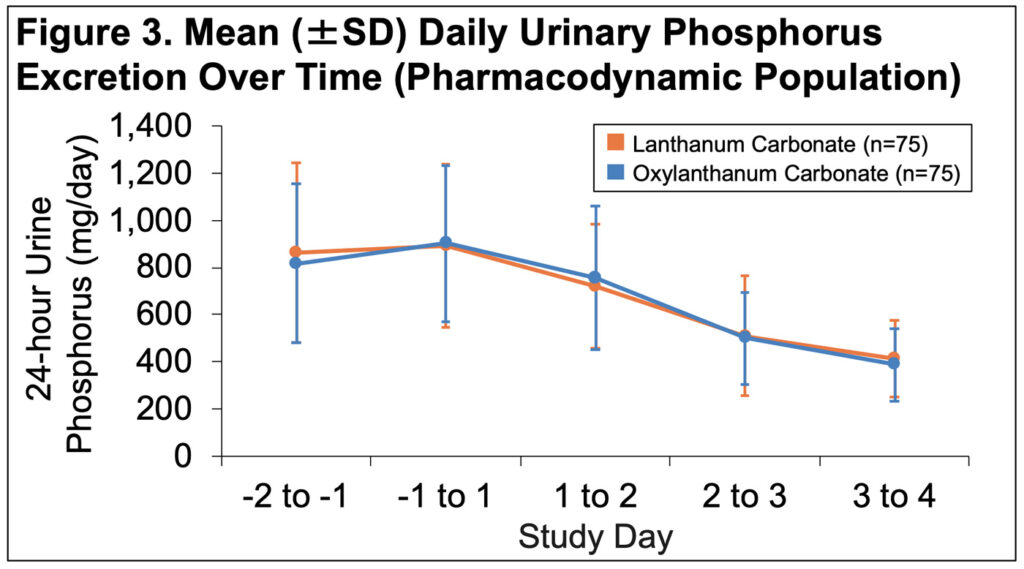
- Mean daily P declined over time to a similar extent in both groups(Figure 3)
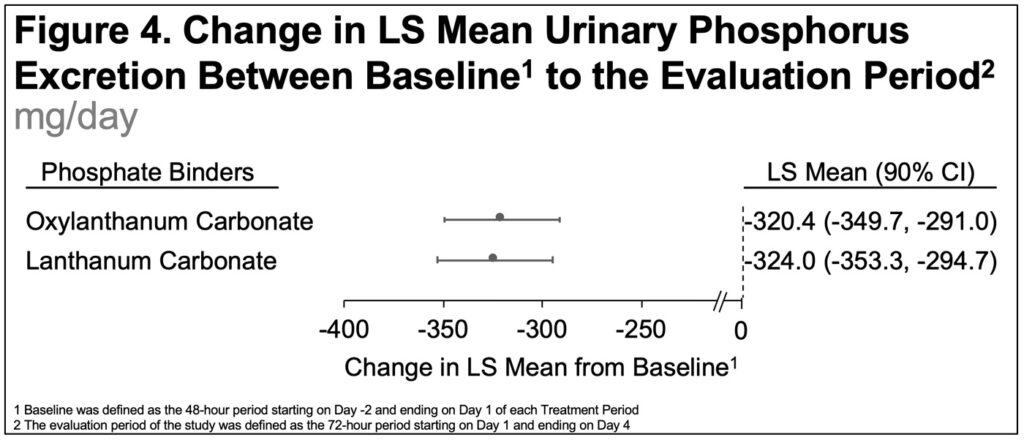
- The change from BL to the Evaluation Period in the LS mean 24-hour urine P excretion was -320.4 mg/day for OLC and -324.0 mg/day for LC(Figure 4)
- The 90% CI for the LS mean change in urinary P excretion from BL (treatment difference) was (-37.8, 45.1), which was entirely contained within the predefined ±20% acceptance range of (-64.8, 64.8)(Figure 5)
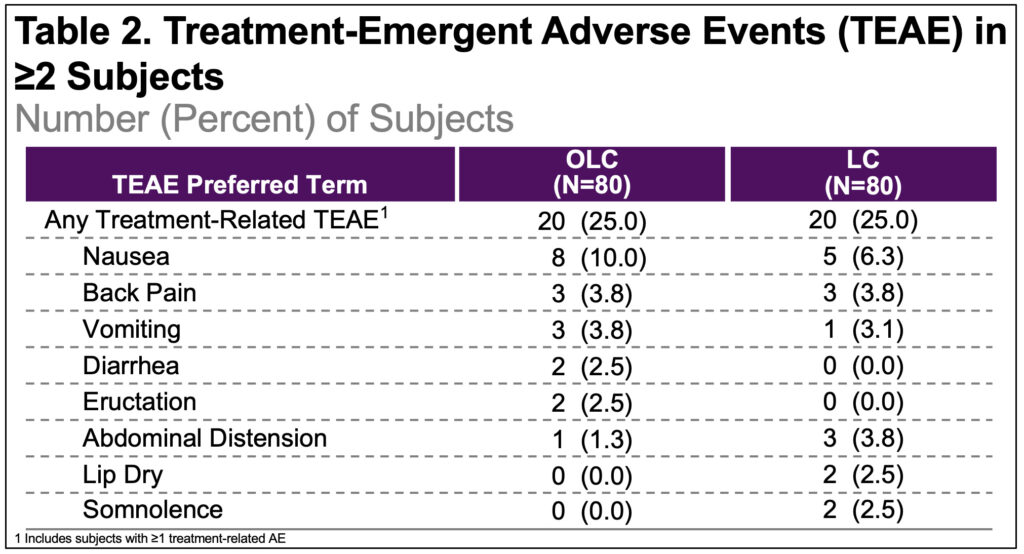
- Incidence of treatment-emergent adverse events (TEAEs), regardless of relatedness to the study drugs was 35% in both groups; related adverse events (AE) were reported in 25% in each group(Table 2)
- No AEs resulted in treatment discontinuation and none were serious/severe

CONCLUSIONS
- In this randomized, crossover study, both OLC and LC increased urinary P excretion similarly and were found to be bioequivalent
- Declines in daily urinary phosphorus excretion after treatment were similar for OLC and LC
DISCUSSION
- Although the majority of patients with ESKD in the United States are managed with phosphate binders, the optimal strategies for garnering the greatest effectiveness from these drugs while minimizing pill burden and adverse effects for patients are yet to be defined
- The higher intrinsic phosphate binding capacity of lanthanum without the potential variability introduced by the need to completely chew tablets may provide an additional option for patients with hyperphosphatemia for whom chewing tablets is disliked, inconvenient, or difficult
References:
1.References:
1.Lv JC. et al., Adv Exp Med Biol. 2019. Aug.
2.K/DOQI. Am J Kidney Dis. 2003. Oct.
3.KDIGO. Kidney Int Suppl. 2017. Jul.
4.McClure ST, et al., Nutrients. 2017. Jan.
Acknowledgments:
Writing support was provided by Xelay Acumen Group, Inc., and funded by Unicycive, Inc.